Anxiety is widely conceptualized as a state of heightened distress, arousal, and vigilance that can be elicited by potential threat (
1,
2). When extreme or pervasive, anxiety can be debilitating (
3). Anxiety disorders are among the leading cause of years lived with disability, afflicting ∼300 million individuals annually (
3). In the United States, nearly 1 in 3 individuals will experience an anxiety disorder in their lifetime, diagnoses and service utilization are surging among young people, and direct health care costs exceed $40 billion annually (
3–
6). Yet existing treatments are inconsistently effective or are associated with significant adverse effects, underscoring the urgency of developing a clearer understanding of the underlying neurobiology (
7,
8).
Perturbation and recording studies in rodents and monkeys have begun to reveal the specific molecules and microcircuits that control defensive responses to a variety of threats (
9), but the relevance of these discoveries to the complexities of the human brain and human anxiety is unclear. Human neuroimaging research provides an opportunity to address this translational conundrum. Clinical studies of anxiety have leveraged a variety of experimental challenges—from aversive photographs and other symptom provocations to threat conditioning and trauma recall paradigms—to identify aspects of brain function that discriminate individuals with pathological anxiety from control subjects. Preclinical human studies of anxiety have taken a different tack and narrowly focused on tracing the circuits normatively engaged by the anticipation of potential threat in nominally healthy samples. Preclinical studies are essential for understanding how anxiety normally works, free from the confounders, comorbidities, and sequelae of psychiatric disease and treatment. They provide a translational bridge to mechanistic studies in animals, which also tend to focus on adaptive behavioral responses (e.g., freezing) to threat. And because they capture symptoms and intermediate phenotypes—such as subjective feelings of anxiety—that cut across disorders, human preclinical studies provide a unique opportunity to develop transdiagnostic biomarkers (
10,
11). While clinical and preclinical studies both provide valuable clues about the neural underpinnings of anxiety, as the literature has grown, it has become increasingly difficult to integrate the two veins of research into a unified conceptual framework.
In this issue of the
Journal, Chavanne and Robinson (
12) provide the most comprehensive coordinate-based meta-analysis of anxiety-related functional neuroimaging research in over a decade (
13), focusing on studies of emotion perception and provocation (156 studies with 693 preclinical participants, 2,554 case subjects, and 2,348 control subjects). Their clinical meta-analyses included patients with generalized anxiety disorder, social anxiety disorder, specific phobia, panic disorder, posttraumatic stress disorder, and mixed anxiety diagnoses. Preclinical analyses included coordinates culled from a variety of unpredictable or uncertain threat studies (e.g., threat of shock). Notably, the authors have made their raw data (
https://osf.io/9s32h) and meta-analytic maps (
https://neurovault.org/collections/6012) freely available, facilitating a range of applications by other investigators.
The publication of Chavanne and Robinson’s report provides an opportune moment to take stock of what we have learned from 20 years of anxiety-related neuroimaging research and to identify the most fruitful next steps.
Chavanne and Robinson show that clinical anxiety is associated with heightened reactivity in an extended subcortico-cortical circuit. Subcortically, this encompasses several regions implicated in animal models of anxiety, including regions of the amygdala, anterior hippocampus, and periaqueductal gray (
9). The bed nucleus of the stria terminalis (BST)—another key player in animal models of anxiety that has only recently begun to attract the attention of the psychiatric imaging community (
9,
14)—was also evident in secondary analyses that excluded medicated patients. In the cortex, Chavanne and Robinson show that clinical anxiety is associated with elevated reactivity in the dorsolateral prefrontal cortex, pregenual anterior cingulate cortex, midcingulate cortex, and anterior insula. Collectively, these observations replicate and extend Etkin and Wager’s influential 2007 neuroimaging meta-analysis (
13), which identified heightened amygdala and insula reactivity as a potential “final common pathway” for pathological anxiety.
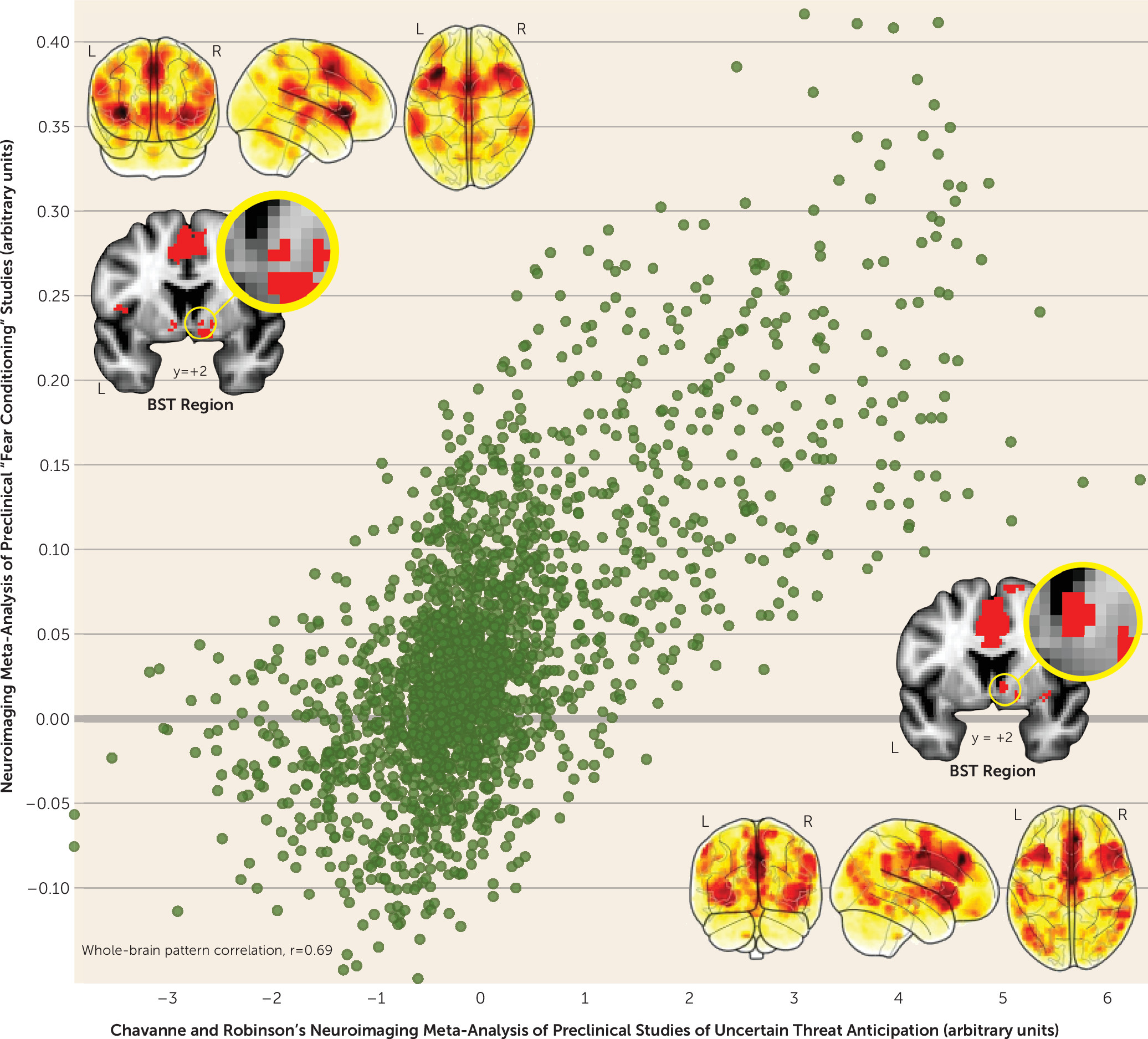
A key feature of Chavanne and Robinson’s report is the systematic analysis of preclinical studies of anxiety. This revealed a circuit encompassing many of the regions identified by their clinical analyses—including the BST, periaqueductal gray, midcingulate cortex, and anterior insula—an observation consistent with models suggesting that pathological anxiety reflects sensitization of the circuitry responsible for orchestrating normative states of anxiety (
1,
2). Chavanne and Robinson also provide exciting new evidence that uncertain and certain threat recruit an overlapping core network in humans (
Figure 1). Since the time of Freud, the heuristic distinction between uncertain (“anxiety”) and certain (“fear”) threat has been a hallmark of neuropsychiatric models of emotion (
15), including the National Institute of Mental Health’s Research Domain Criteria framework, but the underlying neurobiology has remained contentious (
16). Leveraging data from Fullana and colleagues’ recent meta-analysis of preclinical “fear conditioning” studies (
17)—the prototypical laboratory assay of “fear”—Chavanne and Robinson show that certain and uncertain threat are processed in co-localized regions of the periaqueductal gray, BST, midcingulate cortex, and anterior insula, a finding that neatly dovetails with other recent work in humans and rodents (
9,
16). These observations reinforce claims that “anxiety” and “fear” are more biologically alike than different and reflect the operation of a shared set of neural building blocks (
16).
Remarkably, the amygdala was not evident in either Chavanne and Robinson’s or Fullana and colleagues’ preclinical meta-analytic results (
Figure 1). Why? It may reflect the systematic exclusion of studies that relied on popular small-volume significance thresholds and region-of-interest approaches. It may reflect systematic differences in the kinds of tasks used in preclinical and clinical anxiety research (e.g., shock anticipation versus emotional faces). Or it may simply reflect insufficient power to declare modest effects “whole-brain significant” in individual studies (i.e., the median sample size was <30). Consistent with this possibility, preclinical studies using larger samples and improved techniques for data acquisition and processing have shown that the dorsal amygdala (in the region of the central nucleus) is engaged by uncertain
and certain threat anticipation, consistent with perturbation studies in animals (
9,
16).
In sum, two decades of neuroimaging research demonstrate that anxiety disorders are associated with exaggerated reactivity to emotional challenges in regions of the amygdala, BST, periaqueductal gray, midcingulate cortex, and anterior insula. This extended circuit is recruited by uncertain (“anxiety”) and certain (“fear”) threat in nominally healthy individuals, suggesting that clinical and preclinical studies are tapping a common process. The subcortical components of this core anxiety circuit show an encouraging degree of convergence with those implicated by mechanistic work in rodents, monkeys, and humans (
1,
9). Furthermore, Chavanne and Robinson’s careful follow-up analyses make it unlikely that these results are an artifact of publication biases.
Nevertheless, Chavanne and Robinson’s report serves as a sober reminder that most of the work necessary to understand the brain bases of human anxiety remains undone. Anxiety is a multifaceted construct that includes alterations in subjective distress, cognition, arousal, and behavior that cut across multiple timescales and disorders (
1,
2,
18). It is unclear how the extended anxiety circuit relates to these narrower facets and whether particular circuit components or their functional interactions causally contribute to the development and maintenance of psychopathology. It is also unclear whether hyperreactivity in this circuitry is specific to anxiety disorders or extends more broadly to encompass the internalizing spectrum. In the specific case of the amygdala, the latter appears to be true (
19,
20), an observation consistent with the efficacy of antidepressants for both depression and most anxiety disorders (
7,
8).
Although diagnostic differences are possible, Chavanne and Robinson acknowledge that more data are needed. At present, valid inferences are thwarted by the limited number of studies available for many diagnoses and by differences in both power and paradigm across diagnoses. Indeed, their meta-analyses included ∼3 times more studies of social anxiety disorder (k=41) than panic disorder (k=14), with systematic differences in the tasks used to probe particular diagnoses (e.g., emotional faces versus symptom provocation). The hazard of glossing over diagnosis-task confounders is underscored by Chavanne and Robinson’s supplementary meta-analyses of cognitive tasks, which revealed radically different correlates of pathological anxiety compared with those evident for emotion tasks (e.g., midcingulate cortex hyporeactivity in patients). Widespread comorbidity and inadequate diagnostic reliability further muddle matters (
21,
22). Along these lines, it is also unclear whether observed differences in BST reactivity across Chavanne and Robinson’s meta-analyses reflect differences in population, power, or paradigm. These limitations are not specific to Chavanne and Robinson’s study; they cut across much of the literature and afflict other recent meta-analyses (
19,
20).
Overcoming these challenges will require larger and more diagnostically diverse samples and an emphasis on more reliable dimensional approaches (
21,
22). The development and application of tasks optimized for theory-driven computational modeling would provide important opportunities for understanding the mechanisms that promote extreme anxiety in humans (e.g., aberrant processing of risk or threat ambiguity), clarifying the functional contribution of specific components of the extended anxiety circuit (e.g., BST versus midcingulate cortex), and facilitating translation between human and animal research (
23).
Chavanne and Robinson’s observations raise the possibility that hyperreactivity of the extended anxiety circuit could be used as an objective transdiagnostic biomarker. Determining whether this neural “signature” possesses the requisite reliability, sensitivity, and specificity will require more sophisticated machine learning approaches and larger, more diverse samples (
10,
11). Development of anxiety-related biomarkers has the potential to enable preclinical tests of target engagement and to expedite the development of new treatments. To the extent that biomarker development is centered on a dimensional outcome that cuts across disorders, like anxious distress, it will be important to demonstrate generalizability across elicitors and tasks.
Two decades of human neuroimaging research have yielded steady advances in our understanding of the neural systems underlying adaptive and maladaptive anxiety. Chavanne and Robinson’s observations highlight the relevance of a core circuit encompassing a mixture of the “usual suspects” (amygdala and anterior insula) and some less familiar actors (BST, periaqueductal gray, and midcingulate cortex). Despite this progress, the mechanisms that cause pathological anxiety remain uncertain, and existing treatments remain far from curative for many. Addressing these challenges will require an increased investment in anxiety research, one commensurate with the staggering burden that anxiety disorders impose on global public health, and an enhancement of coordination of human and animal research. The latter could be achieved by combining perturbation techniques in animals with the same neuroimaging strategies routinely used in humans, enabling the development of bidirectional translational models (
9). Nonhuman primate models are likely to be especially informative for understanding cortical components of the extended anxiety network, given uncertain or absent anatomical homologies in rodents (
24). Finally, a greater emphasis on dimensional phenotypes and computational approaches promises to further accelerate efforts to alleviate the suffering caused by pathological anxiety.
Acknowledgments
The authors acknowledge assistance from K. DeYoung, L. Friedman, and J. Smith.