Patient Disposition and Baseline Characteristics
Among 200 patients enrolled and randomized, 196 received blinded study drug (zuranolone 50 mg/day, N=98; placebo, N=98) (
Figure 1). Four patients were randomized but not treated because of patient withdrawal from the study (one patient in the zuranolone group and two in the placebo group) or loss to follow-up (one patient in the placebo group). Most patients completed the study treatment (zuranolone group, 90.8%; placebo group, 92.9%), and the proportion of patients who prematurely discontinued study treatment was similar between treatment groups (zuranolone group, 9.2%; placebo group, 7.1%). Similarly, most patients who received study drug completed the 45-day study (zuranolone group, 85.7%; placebo group, 87.8%).
Demographic and baseline clinical characteristics were generally balanced between treatment groups (
Table 1). Overall, 21.9% of patients were Black/African American and 38.3% were Hispanic/Latina. The mean age was 30.0 years (SD=5.9) in the zuranolone group and 31.0 years (SD=6.0) in the placebo group. Most patients experienced onset of depression within 4 weeks postpartum (zuranolone group, 65.3%; placebo group, 68.4%). Most patients had a baseline HAM-A score ≥20 (zuranolone group, 75.5%; placebo group, 78.6%), indicating moderate to severe anxiety (
19). Baseline stable antidepressant use was similar between the zuranolone and placebo groups (15.3% each). Among patients using concomitant psychotropic medications, most took selective serotonin reuptake inhibitors (zuranolone group, 68.2%; placebo group, 94.1%), including sertraline (zuranolone group, 40.9%; placebo group, 70.6%) and escitalopram (zuranolone group, 13.6%; placebo group, 11.8%).
Efficacy Outcomes
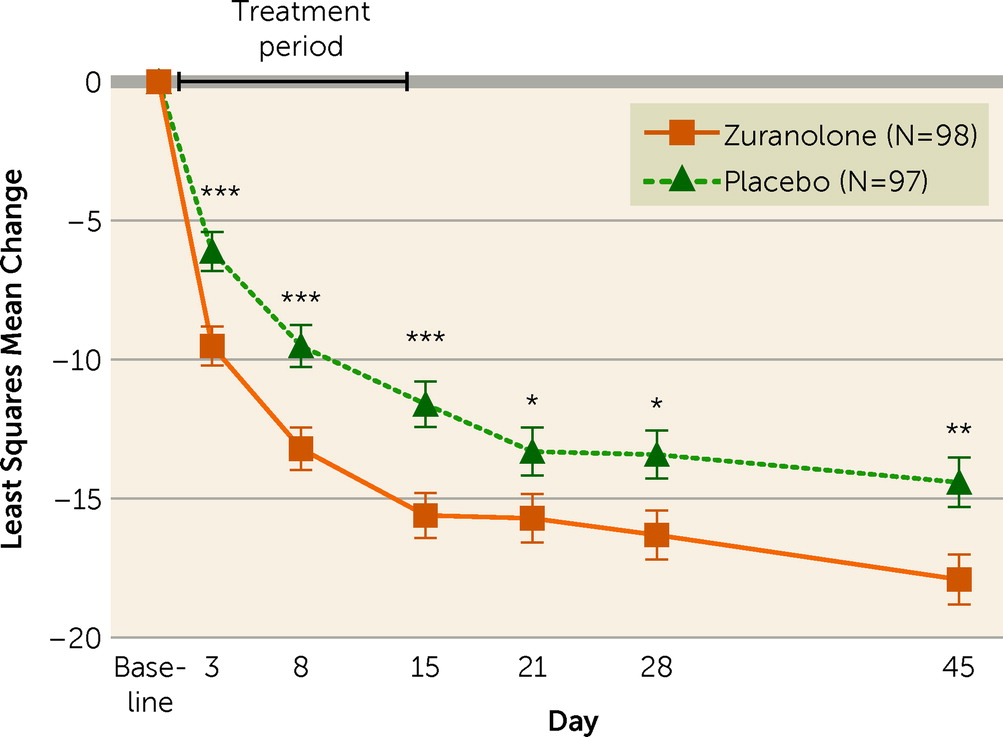
The study demonstrated statistically significant improvement in depressive symptoms on the primary endpoint, as assessed by change from baseline in HAM-D score at day 15, in the zuranolone group compared with the placebo group (least squares mean [LSM]=−15.6, SE=0.82, vs. LSM=−11.6, SE=0.82; LSM difference=−4.0, 95% CI=−6.3, −1.7; p=0.001; Cohen’s d=0.52) (
Figure 2; see also Tables S1 and S2 in the
online supplement). The study also demonstrated statistically significant improvements on the key secondary endpoints in the zuranolone group compared with the placebo group. The zuranolone group demonstrated a significantly greater change from baseline in HAM-D score compared with the placebo group at day 3 (LSM=−9.5, SE=0.70, vs. LSM=−6.1, SE=0.71; LSM difference=−3.4, 95% CI=−5.4, −1.4; p=0.001; Cohen’s d=0.45), day 28 (LSM=−16.3, SE=0.88, vs. LSM=−13.4, SE=0.88; LSM difference=−2.9, 95% CI=−5.4, −0.5; p=0.02; Cohen’s d=0.33), and day 45 (LSM=−17.9, SE=0.90, vs. LSM=−14.4, SE=0.90; LSM difference=−3.5, 95% CI=−6.0, −1.0; p=0.007; Cohen’s d=0.33) (see Tables S1 and S2 in the
online supplement). Similarly, change from baseline in CGI-S score at day 15 was also significantly greater in the zuranolone group compared with the placebo group (LSM=−2.2, SE=0.14, vs. LSM=−1.6, SE=0.14; LSM difference=−0.6, 95% CI=−0.9, −0.2; p=0.005) (see Table S1 in the
online supplement).
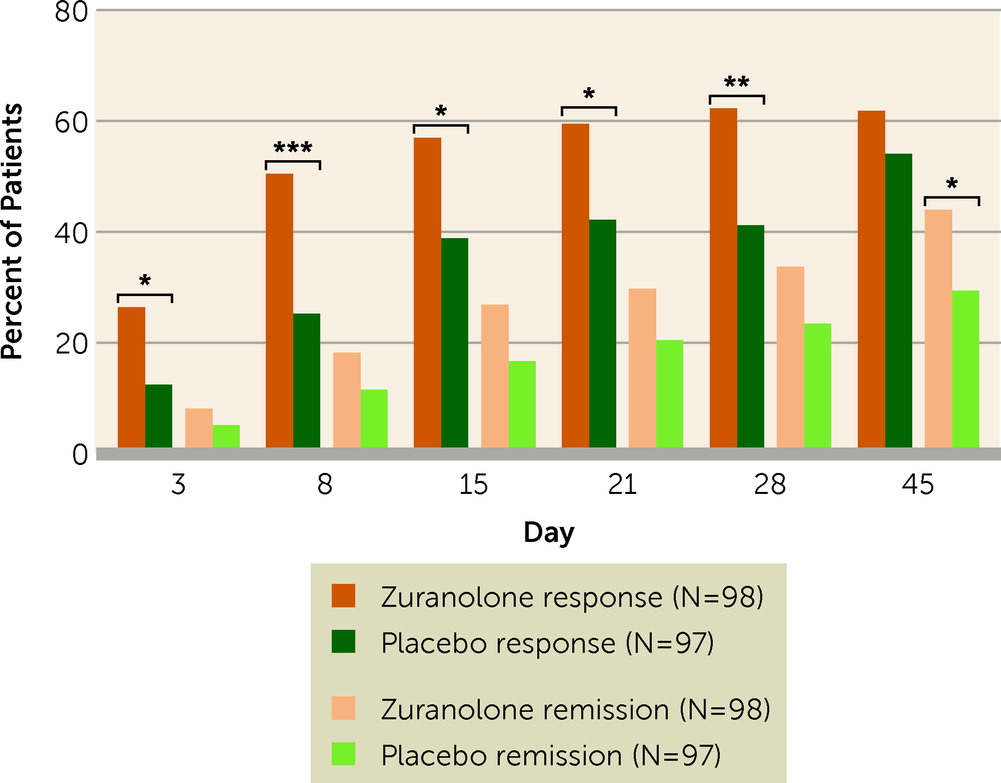
Other secondary endpoint results supported the primary and key secondary endpoints. Response to zuranolone treatment was rapid, with a median time to first HAM-D response of 9 days in the zuranolone group and 43 days in the placebo group. The proportion of patients achieving HAM-D response favored zuranolone compared with placebo starting at day 3, and this trend was observed at every subsequent study visit (see Table S3 in the
online supplement). The proportion of patients achieving HAM-D response at day 15 was significantly higher in the zuranolone group compared with the placebo group (57.0% [N=53] vs. 38.9% [N=35]; odds ratio=2.02, 95% CI=1.11, 3.67; p=0.02) (
Figure 3). The HAM-D remission rate was numerically greater for zuranolone compared with placebo treatment at day 15 (26.9% [N=25] vs. 16.7% [N=15]; odds ratio=1.78, 95% CI=0.88, 3.62; p=0.11) and significantly greater at day 45 (44.0% [N=37] vs. 29.4% [N=25]; odds ratio=2.08, 95% CI=1.11, 3.92; p=0.02) (see Table S3 in the
online supplement). Consistent with the primary and key secondary endpoints showing overall improvements in total score on the HAM-D at day 15, the majority of change from baseline in individual HAM-D item scores (15 of 17 items) and all HAM-D subscale scores favored zuranolone compared with placebo (see Figures S1 and S2 in the
online supplement). Improvements in depressive symptoms with zuranolone as assessed by change from baseline in HAM-D total score were observed irrespective of concomitant antidepressant use from baseline (see Table S4 in the
online supplement). The day-15 CGI-I response rate was significantly greater in the zuranolone group compared with the placebo group (66.7% [N=62] vs. 46.7% [N=42]; odds ratio=2.23, 95% CI=1.22, 4.07; p=0.009). Improvement in depressive symptoms as assessed by change from baseline MADRS score was also significantly greater in the zuranolone group (LSM=−19.7, SE=1.20) compared with the placebo group (LSM=−14.6, SE=1.21) at day 15 (LSM difference=−5.1, SE=1.71, 95% CI=−8.4, −1.7; p=0.003) and at days 8 (p=0.004) and 45 (p=0.01) (see Figure S3 in the
online supplement). Improvements in anxiety as assessed by change from baseline HAM-A score at day 15 were also significantly greater in the zuranolone group compared with the placebo group (LSM=−12.8, SE=0.69, vs. LSM=−10.6, SE=0.70; LSM difference=−2.2, SE=0.98, 95% CI=−4.2, −0.3; p=0.02) (see Figure S4 in the
online supplement).
Consistent with investigator-reported outcome measures, patient-reported change from baseline EPDS scores was significantly greater in the zuranolone group compared with the placebo group at day 3 (LSM=−3.8, SE=0.49, vs. LSM=−2.3, SE=0.49; LSM difference=−1.5, 95% CI=−2.9, −0.1; p=0.03), day 8 (LSM=−8.4, SE=0.60, vs. LSM=−6.2, SE=0.59; LSM difference=−2.2, 95% CI=−3.8, −0.5; p=0.01), day 15 (LSM=−10.3, SE=0.66, vs. LSM=−8.4, SE=0.66; LSM difference=−2.0, 95% CI=−3.8, −0.1; p=0.04), and day 45 (LSM=−12.2, SE=0.76, vs. LSM=−9.8, SE=0.76; LSM difference=−2.4, 95% CI=−4.5, −0.3; p=0.03) (see Figure S5 in the online supplement). Significant improvements in patient-reported PHQ-9 scores in the zuranolone group compared with the placebo group were reported at days 8 and 15, with numerically lower scores at all other time points except at day 3 (see Figure S6 in the online supplement).
Safety and Tolerability
Zuranolone was generally well tolerated; most patients who experienced treatment-emergent adverse events reported mild or moderate events (
Table 2). During the 14-day treatment course, treatment-emergent adverse events were reported by 60.2% (N=59) and 41.8% (N=41) of patients receiving zuranolone and placebo, respectively. In the zuranolone group, 16.3% (N=16) experienced treatment-emergent adverse events leading to dosage reduction, compared with 1.0% (N=1) in the placebo group; 15 of these patients (zuranolone, N=14; placebo, N=1) completed the study. Treatment-emergent adverse events leading to dosage reduction in more than one patient receiving zuranolone included somnolence (7.1%, N=7), dizziness (6.1%, N=6), and sedation (3.1%, N=3); the treatment-emergent adverse event leading to dosage reduction in the placebo group was anxiety. Among patients who experienced treatment-emergent adverse events leading to discontinuation of study drug, 4.1% (N=4) were in the zuranolone group and 2.0% (N=2) were in the placebo group.
The most common treatment-emergent adverse events (≥5%) reported by patients were somnolence (zuranolone, 26.5%; placebo, 5.1%), dizziness (zuranolone, 13.3%; placebo, 10.2%), sedation (zuranolone, 11.2%; placebo, 1.0%), headache (zuranolone, 9.2%; placebo, 13.3%), diarrhea (zuranolone, 6.1%; placebo, 2.0%), nausea (zuranolone, 5.1%; placebo, 6.1%), urinary tract infection (zuranolone, 5.1%; placebo, 4.1%), and COVID-19 (zuranolone, 5.1%; placebo, 0%). Two serious adverse events were reported, both of them in patients in the zuranolone group—one during the treatment course and one during the posttreatment period—and were considered unrelated to study drug. No loss of consciousness and no clinically significant changes in vital signs, ECG, or clinical laboratory parameters were reported. Most patients had maintenance or improvement from baseline in maximum severity score on the C-SSRS at all postbaseline time points. The change from baseline in PWC-20 scores was similar between treatment groups, with no evidence of withdrawal symptoms. During the follow-up period, two patients in the zuranolone group and one in the placebo group started new antidepressant treatments. No patient deaths occurred in the study.