Depression post-TBI has been associated with extensive adverse outcomes such as impaired psychosocial functioning, impaired cognitive performance, and increased risk of other psychiatric disorders, such as anxiety disorders (
3). It is also more common for those with post-TBI depression to experience greater postconcussive symptoms (headaches, sleep disturbances, cognitive and memory impairment) than those who do not develop depression following a TBI (
4). The heightened need for research into depression post-TBI is highlighted by Bombardier (
5), who found 53.1% of 559 TBI patients developed major depressive disorder (MDD) within 1 year. Therefore, early treatment of depression is crucial to meet the needs of the high proportion of post-TBI patients affected by it.
The evidence for the effectiveness of pharmacotherapy for depression in the TBI population is conflicted and equivocal, with no overall trend extrapolated. Many studies within the current literature lack methodological rigor, with limited sample sizes and nonrandomized control groups. Currently, the first-line treatment of depression post-TBI is selective serotonin reuptake inhibitors (SSRIs), which may be attributed to the evidence base surrounding their use in MDD. SSRIs display a safer and more tolerated side effect profile than tricyclic antidepressants (TCAs). However, the efficacy of different SSRIs and TCAs varies. TCAs present an amplified risk of toxicity in overdose and side effects such as sedation and urinary retention; therefore, they are largely a second-line intervention. Findings by Jorge and Arciniegas (
6) and Fann et al. (
7) have suggested that SSRIs are the most effective pharmacotherapy in treating depression post-TBI. However, because of limited evidence and inconsistencies in the quality of research, they could not establish clinical guidelines on their use. This again emphasizes the need for more statistically powerful, placebo-included randomized controlled trials to validate this association, which can further inform the delivery of high-quality evidence recommendations.
Alternative pharmacotherapy options, such as monoamine oxidase inhibitors (MAOIs) and serotonin noradrenaline reuptake inhibitors (SNRIs), are less established but may offer new avenues of treatment. These are not often used because of the strict guidelines the patient must adhere to while taking them, including strict diet restriction. This is problematic in the TBI population, as the common cognitive consequences often affect medication compliance. SNRIs have a dual action on serotonin and noradrenaline reuptake inhibition and could have potential. However, this class of antidepressants is currently considered a second- or third-line treatment in MDD, and there is limited evidence for their use specifically for depression post-TBI. This highlights the critical need for more comprehensive research into the potential effectiveness of SNRIs and MAOIs for treating depression post-TBI.
Anticonvulsants such as lamotrigine have also shown specific benefit in treating the depressive symptoms in bipolar disorder, which may be generalized to future research into its potential in MDD (
10). There is a larger evidence base for the use of anticonvulsants such as valproate, carbamazepine, and phenytoin in treating other neuropsychiatric sequelae of TBI, including aggression, agitation, and behavioral issues. However, there are also contradictory results from Smith et al. (
11), who showed that they produced negative effects on cognition and motor performance.
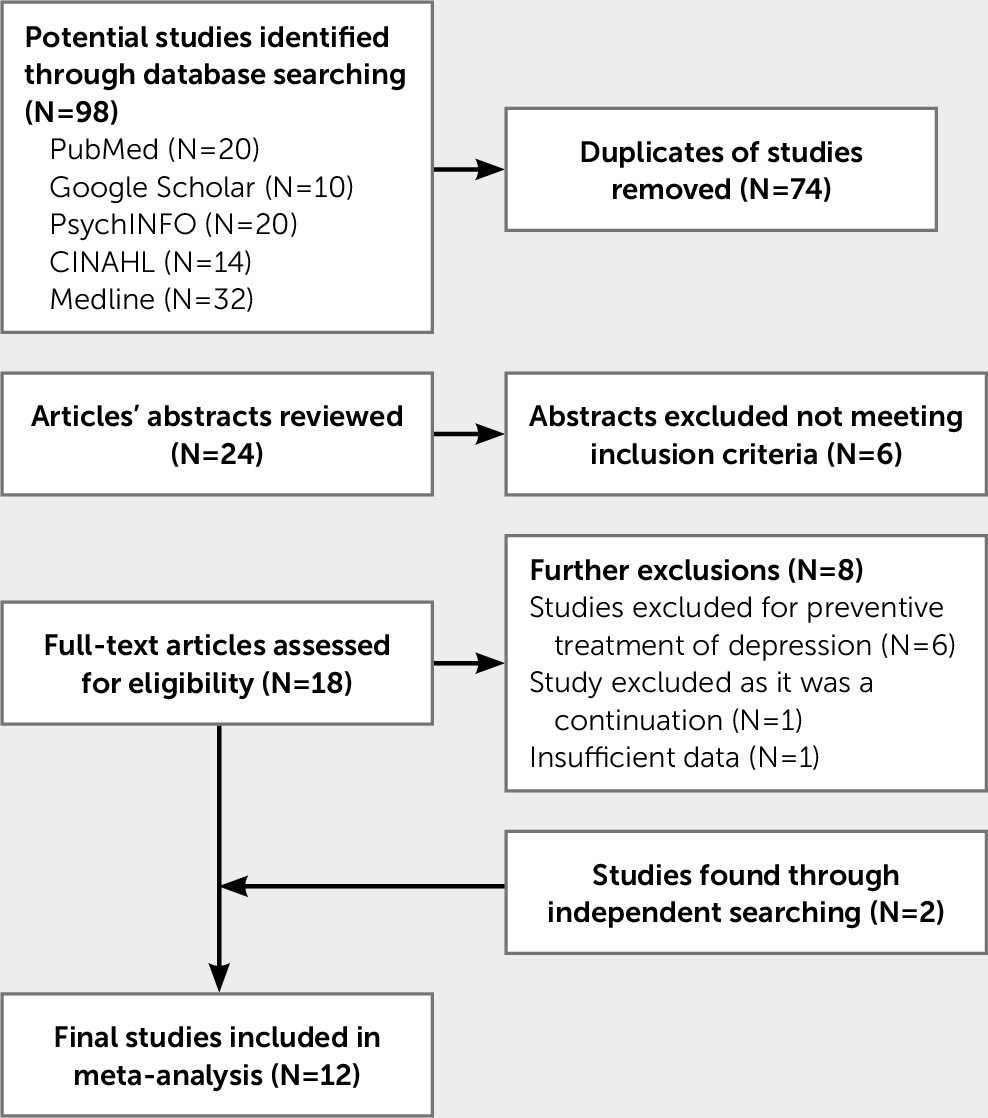
Overall, the current studies examining the treatment for depression post-TBI all implemented different outcome measures, sample sizes, periods of treatment, TBI severity, and time between TBI and the onset of depression. This makes comparison across studies especially difficult. There is a need to amalgamate the current research to provide clear guidelines that inform the treatment of depression following TBI. The aim of the current meta-analysis is to converge existing studies and provide a synthesized effect size to measure the effectiveness of pharmacotherapy for depression following a TBI.
Discussion
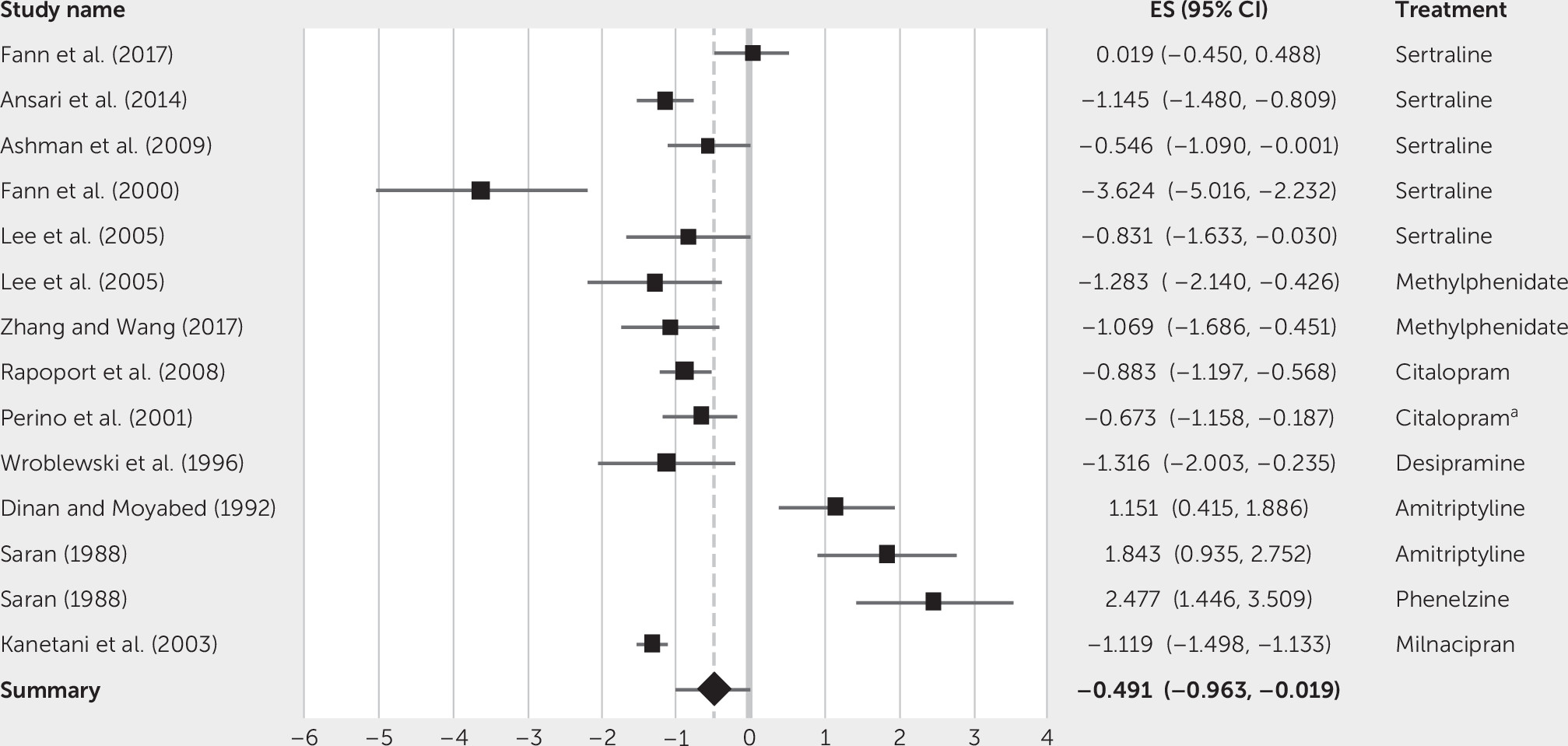
These results indicate that pharmacological treatment may be mildly to moderately effective in treating depression post-TBI. Specifically, treatments such as citalopram, sertraline, and methylphenidate may be effective in reducing depressive symptoms in patients with TBI. The overall small to moderate effect size (Cohen’s d=–0.49) suggests that there is a significant reduction in symptoms from pre- to postintervention scores. However, this overall result should be interpreted with caution, because there is a lack of evidence in the form of large randomized controlled trials but rather includes a small sample of studies with poor methodological quality.
The findings from the current meta-analysis are supported by a previous meta-analysis (
28) that found similar results. They evaluated antidepressant treatment effectiveness in nine studies treating depression, finding significant effects in favor of antidepressants. However, the authors could not draw definite conclusions on treatment efficacy due to the limited number of studies available in the literature.
This stance is reflected in another meta-analysis conducted by Barker-Collo et al. (
22), who analyzed both pharmacological and nonpharmacological treatments and found that treatment did decrease depressive symptoms. They came to a similar conclusion that there is insufficient evidence to conclusively recommend treatment options to TBI patients. The current meta-analysis does offer an expansion on the included literature but still contains limitations; interpretation of the results should acknowledge this.
While the current meta-analysis was under review, the authors were made aware of a newly published meta-analysis by Kreitzer et al.(
29). Although the overall conclusions slightly differ, the results of both meta-analyses are very similar. This is encouraging from a scientific replicability standpoint. For example, Kreitzer et al. (
29) acknowledged significant reductions in depression scores for individuals after pharmacotherapy (mean change in HADS scores of –11.2). However, similar to the current meta-analysis, Kreitzer et al. (
29) found no significant reduction in depression scores when considering the evidence from randomized controlled trials alone, for which Kreitzer et al. (
29) derived their conclusion. In contrast, the current meta-analysis derived its conclusion from the entire evidence base, including both randomized controlled trials and single-group design studies, which indicated a small to medium effect for pharmacological treatments for depression following TBI. In addition to an overall omnibus effect size for all studies, the current meta-analysis also provided separate effect sizes for both single-group design studies and randomized controlled trials for the reader’s consideration.
The most consistent modest effect size was for sertraline, followed by methylphenidate and citalopram. A pooled estimate calculated for the sertraline studies (Cohen’s d=–1.02) produced a large effect size, even with the included outlier. Therefore, these findings suggest that sertraline, with the largest number of studies included and effect size, is potentially the most efficacious pharmacotherapy in treating depression after a TBI. Furthermore, the results from methylphenidate and milnacipran could have further implications in guiding potential pharmacological options, especially with the additional benefits on cognitive impairments and tolerability.
The studies that had nonsignificant effect sizes consisted of control groups such as in Fann et al. (
19). In their paper, Fann et al. (
19) discussed several possibilities for such nonsignificant effect sizes. One hypothesis is that the control group had more contact with staff, which may have produced the reduction in depressive symptoms. Fann et al. (
19) highlighted the high prevalence of social isolation in the TBI population and hypothesized that frequent contact with health care staff might have reduced social isolation and decreased depression scores. This could be important in the context of rehabilitation; researching what nonpharmacological mechanism caused reductions in reported depression could potentially lead to subsequent application in nonpharmacological therapies.
In comparison, a recent study by Cipriani et al. (
30) investigating the efficacy of 21 different antidepressants in treating MDD showed modest effect sizes for all antidepressants in reducing depressive symptomology. Although this exhibits a significant difference in response compared with depression post-TBI, the study drew from a substantial evidence base, which is severely lacking in regard to the TBI population. Consequently, it is likely that similar results of treatment efficacy may be found if there is further comprehensive research into depression post-TBI.
In contrast, Saran (
21) found that both amitriptyline and phenelzine had greater effects in reducing HAM-D scores in those without a minor closed head injury. Similarly, Dinan and Moyabed (
18) reported an 85% response to amitriptyline in those with just MDD, compared with 31% responding to treatment in those with depression post-TBI. Cipriani et al. (
29) found that amitriptyline produced the greatest effect size in non-TBI MDD, which is interesting compared to the response to amitriptyline in TBI patients. These findings indicate that depression in TBI may respond differently to treatment compared with the non-TBI population. However, the studies in the TBI population had very low methodological quality, lacked large sample size, and included a crossover trial, so assumptions cannot be conclusive.
A meta-analysis by Price et al. (
31) assessing treatment of depression in neurological disorders (stroke, Parkinson’s disease, multiple sclerosis, epilepsy, and TBI) found statistically significant effects of antidepressants compared with placebo. The meta-analysis also found a small difference in antidepressant responsiveness in this neurological sample when compared with the non-neurologically depressed population. However, as there was only one TBI study included, these conclusions cannot be applied to the whole TBI population. This was acknowledged by the authors, who stated that lack of trials only permitted evidence on Parkinson’s disease and stroke.
The current meta-analysis evaluated only pharmacological treatments. It is important to note that pharmacological treatments alone are not the only options in treating depression in TBI patients. Future research could investigate medication effectiveness in the setting of other interventions, such as cognitive-behavioral therapy, magnetic field stimulation, and psychoeducation. This is because treating neuropsychiatric sequelae such as depression in TBI patients requires a multifactorial approach to assist TBI patients in their recovery and increase their quality of life.
Although this study considered the proposed effectiveness of the antidepressants, it is also imperative to look at the population to whom the treatment will be administered. Their existing comorbidities and current medication use will inevitably affect which treatment they receive. This is further complicated by the possibility that the characteristics of depression post-TBI and related comorbidities could affect treatment effectiveness. For example, most depressed patients post-TBI also experience executive dysfunction, although the causation for this is not clear-cut (it is due either to the TBI damage or specific symptoms from the depression). Goryln et al. (
32) found that deficits in executive function could influence treatment adherence and predict patients’ response to SSRIs. This also presents the idea of neuropsychological testing to enable identification of those who are at risk of not responding to treatment.
A potential limitation to this meta-analysis is the incorporation of single-group designs. This makes it challenging to delineate the treatment effectiveness from the natural course of depression in the context of TBI rehabilitation and recovery. This is also highlighted by Kreitzer et al. (
29). Incidentally, single-group design studies produced a large pooled effect size (Cohen’s d=–1.35). However, this is limited in the context of the Cochrane recommendations results from nonrandomized studies should be interpreted with caution. This is because it results in a higher risk of selection bias and can produce effect size estimates that indicate the more extreme ends of the effects of treatment than randomized trials do.
Another limitation is the inability to control for the variation in the time since injury or the type and severity of TBI, which otherwise might produce more meaningful results for TBI patients. This undoubtedly contributed to the high heterogeneity, including the different outcome measures and length of treatment. For example, the time from injury varied from 30 days to 18.6 years, and the period of treatment varied from 4 to 30 weeks. The main concern of the shorter administration periods is not allowing for the optimal dosage and adjustment needed for therapeutic levels, such as in the Saran (
21) study. For instance, the SSRI sertraline generally shows a latency period of 2 weeks before it has an effect. Perhaps more significant results could be found if periods of treatment were longer.
A strength of this current meta-analysis is that it has, to the authors’ knowledge, the most up-to-date inclusion and largest compilation of studies. Therefore, this is the largest comprehensive meta-analysis conducted so far on pharmacological treatment options, and not just antidepressants. This can give a wider scope on other pharmacological interventions and their potential effects on depression post-TBI.
To conclude, this meta-analysis suggests that pharmacological treatment may be mildly to moderately effective in treating depression following a TBI. It also demonstrates that sertraline may be the most effective pharmacological treatment option. However, there is still a need for higher quality studies with lower heterogeneity to draw less preliminary and more conclusive inferences concerning TBI treatment. This is important in the context of Fann et al., (
7) who found that untreated depression in TBI patients is associated with poorer psychosocial functioning, cognition, and integration into the community. Consequently, if more comprehensive research is established, especially with pharmacotherapy in conjunction with nonpharmacological therapies, it could help future clinical application and rehabilitation of TBI patients.