A substantial minority of patients who sustain a mild traumatic brain injury (mTBI) develop symptoms that can last for months or years (
1–
3). Emotional distress in the early postinjury period may be the most robust known predictor of symptom persistence after mTBI (
4,
5). Multiple lines of evidence point to a causal relationship, that is, distress contributes to symptom persistence. First, preinjury mental health problems are strongly related to symptom persistence after mTBI (
4,
5). Second, early postinjury emotional distress predicts later disability more so than the reverse temporal pattern (
6). Third, experimentally induced stress heightens postconcussion symptom intensity (
7). The mechanisms by which emotional distress perpetuate symptoms after mTBI are not clear.
One possibility is somatization, which is the process whereby emotional distress manifests as unintentionally produced physical symptoms (
8). Somatization occurs in healthy people, such as noticing an atypical headache after learning that a friend was diagnosed with a brain tumor. Somatization can also exacerbate symptoms of a known disease, such as worsened tremor in Parkinson’s disease (
9). Additionally, somatization is thought to be a common etiological factor in health conditions characterized by nonspecific symptoms, such as irritable bowel syndrome (
10), fibromyalgia (
11), and chronic fatigue syndrome (
12), collectively referred to as functional somatic syndromes (FSSs) (
13,
14). Conceptually, symptoms of stress (e.g., headaches, fatigue, difficulty concentrating, and sleep disturbance) overlap with and could easily be misattributed to mTBI (
7,
15,
16). Individuals who are prone to somatization preinjury may be hypervigilant to physical sensations after mTBI and at risk for attributing benign symptoms to mTBI, exacerbating stress and symptom-focused attention.
Several studies have provided preliminary support for the hypothesis that somatization is a mechanism underlying symptom persistence after mTBI. First, a study of civilian adults with mTBI found that individuals with poor recovery from mTBI reported significantly more “atypical” somatic symptoms not typically associated with mTBI (e.g., gastrointestinal distress, chest pain, and shortness of breath) compared with mTBI patients with good recovery (
17). Second, multiple studies have reported an association between the severity of preinjury nonspecific somatic symptoms and the persistence of postconcussion symptoms after mTBI. In children aged 10–18 years, higher preinjury somatization scores predicted higher postconcussion symptom scores over time (
18). Specifically, females in the highest quartile of preinjury somatization scores reported significantly more postconcussion symptoms at baseline (in the emergency department) and at 2 weeks and 4 weeks postinjury, whereas clinical variables such as loss of consciousness, retrograde or posttraumatic amnesia, and prior concussion history did not predict postconcussion symptom outcomes (
18). In another pediatric study (aged 8–18 years), patients with mTBI who had symptoms for more than one month postinjury had higher parent-rated preinjury scores on a somatic symptom inventory than patients whose symptoms resolved within one month of injury. Higher reported somatization was associated with increased odds (95% CI=1.08–1.69) of experiencing delayed symptom resolution (
19). Similarly, in a study of high school and college athletes, the investigators found that preinjury somatic symptom severity was the strongest preinjury predictor of persistent symptoms after mTBI (
20). In mediation analyses, these authors found that preinjury somatic symptoms affected symptom duration indirectly through its effect on acute postconcussion symptoms (
20). In the only available adult study of this type, predeployment scores on a somatic symptom inventory predicted persistent postconcussion symptoms among military personnel who had sustained a combat-related mTBI (
21).
If somatization is a common mechanism underlying both FSSs and persistent symptoms after mTBI, we would expect that individuals with a history of FSSs who sustain an mTBI might experience more severe and persistent symptoms following that injury. It has already been well established that FSSs tend to co-occur (
22–
24). In the general population, 10% of patients with one FSS will meet diagnostic criteria for another; however, in clinical samples comorbidity rates exceed 50% (
22,
25,
26). For example, the lifetime rates of irritable bowel syndrome co-occurring with other common FSSs were 92% among patients with chronic fatigue syndrome and 77% among patients with fibromyalgia compared with 18% for healthy control subjects (
22). High rates of comorbid chronic fatigue syndrome and fibromyalgia (42%) and fibromyalgia and irritable bowel syndrome (39%) have also been reported (
27). Even though the cardinal symptoms of each FSS seemingly involve distinct bodily systems, patients with one FSS tend to have other FSSs, which suggests a shared etiological factor, such as somatization. Evidence of co-occurrence between FSSs and persistent symptoms following mTBI would support that somatization is also relevant to this condition.
In the present study, we evaluated whether a history of FSSs was related to mTBI outcome by considering two measures of this association. First, we hypothesized that a prior history of FSSs would be overrepresented in a treatment-seeking slow-to-recover mTBI sample. Irritable bowel syndrome, fibromyalgia, and chronic fatigue syndrome occur in 2.4% (
28), 1.5%, and 1.4%, respectively, of people in the general Canadian population (
29). If somatization contributes to both FSSs and persistent symptoms after mTBI, one should see higher rates of preinjury FSSs at mTBI clinic intake. Second, we hypothesized that patients with preinjury FSSs would demonstrate slower or less complete symptomatic recovery from mTBI at the clinical follow-up.
Methods
Participants and Setting
We conducted a secondary data analysis of a knowledge translation intervention involving primary care physicians. For the purposes of this study, participants were collapsed across groups. Participants in the parent study were recruited from two outpatient mTBI specialty clinics in the Greater Vancouver area (British Columbia, Canada) during their first clinic visit. Patients seen in these specialty clinics were treatment-seeking and represented a slow-to-recover group. Participants were eligible for the parent study if they were between 18 and 60 years old, sustained an mTBI within 3 months of the clinic intake, had self-reported fluency in English, and planned to receive follow-up care with a family physician or primary care clinic. To be eligible for the present study, participants were required to have completed the mTBI symptom self-report measure (i.e., the Rivermead Post-Concussion Symptom Questionnaire [RPQ] [
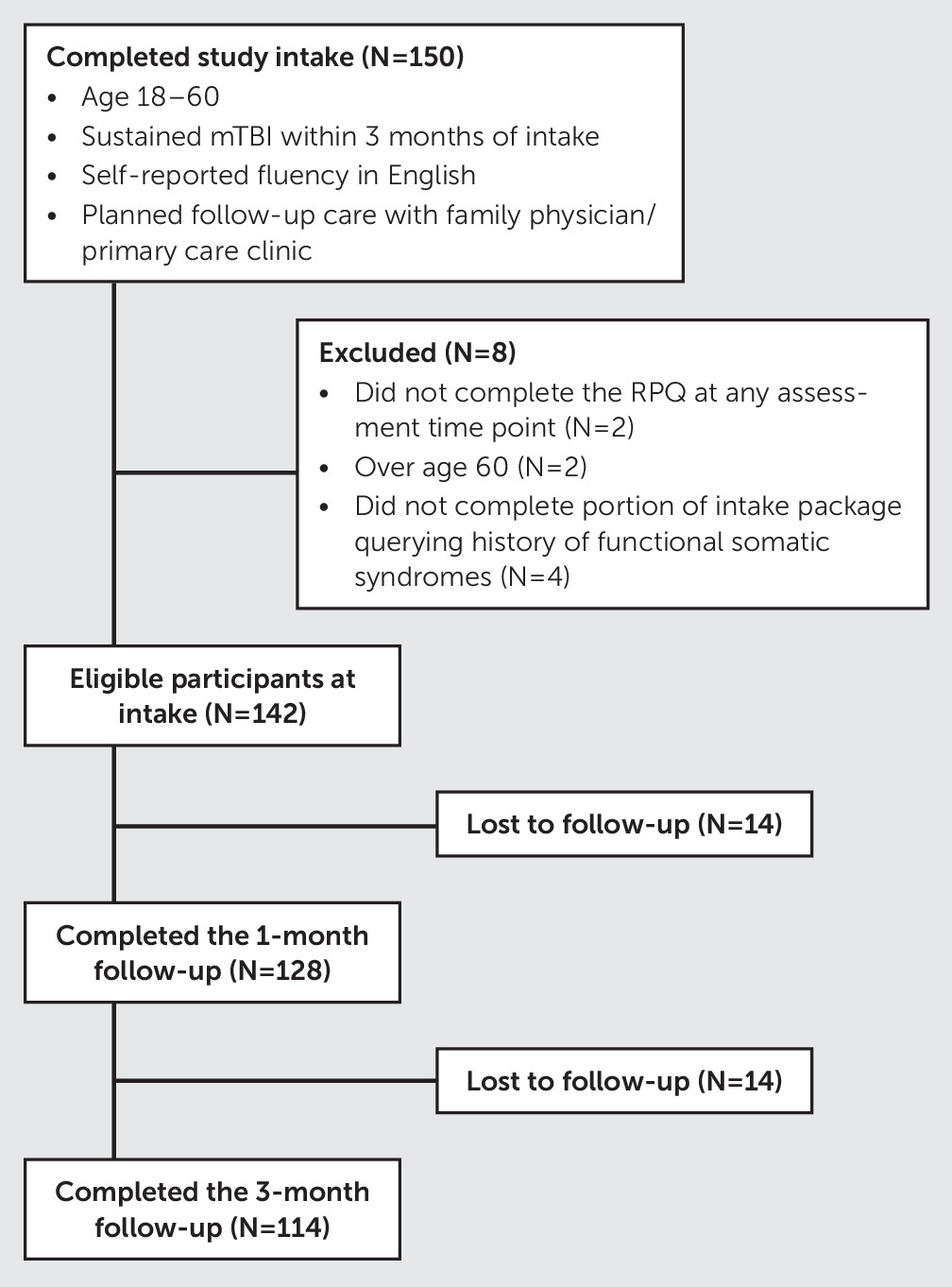
30]) during at least one of the three assessment time points (i.e., clinic intake, 1-month follow-up visit, and 3-month follow-up visit) and to have responded to a sufficient number of questions regarding their history of functional somatic syndromes in order to be classified as having no history of an FSS, a history of one FSS, or a history of two or more FSSs. The participant flow through the study and the number of excluded individuals as well as reasons for exclusion are presented in
Figure 1.
Procedures
Eligible and consenting participants completed study questionnaires in-person at the clinic intake or via the Research Electronic Data Capture (REDCap) (
31) (a secure web-based platform) within the week after clinic intake. Questionnaires were completed again at the 1-month and 3-month follow-up assessments either by telephone or with REDCap.
Measures
At intake, demographic information, injury characteristics, and medical history of the study participants were collected using self-report questionnaires. History of FSSs was surveyed with questions asking whether the participant had ever been diagnosed or treated for irritable bowel syndrome, chronic fatigue syndrome (myalgic encephalomyelitis), or fibromyalgia prior to mTBI. Similar questions regarding history of anxiety and depression were also asked. We collapsed these two items (history and depression) into a single variable (0=neither, 1=history of anxiety or depression) to facilitate comparison with previous studies, which mostly asked about history of mental health problems, and to manage multicollinearity (of 47 participants who reported anxiety and 47 participants who reported depression, 40 reported a history of both).
The severity of mTBI symptoms was assessed with the RPQ, which was administered at the clinic intake, the 1-month follow-up visit, and the 3-month follow-up visit. The RPQ is a 16-item self-report scale that is designed to assess common emotional, behavioral, and physical symptoms of mTBI. Each item is scored on a Likert scale from 0 (“not experienced at all”) to 4 (“a severe problem”), for a total possible score of 64 (
30). Scores of 1 (i.e., “no more of a problem” than before the injury) were not included in the calculation of total RPQ scores, per King et al. (
30).
Statistical Analysis
Demographic characteristics, prior medical history, injury history, and symptom persistence data for patients who completed the initial study visit were evaluated using descriptive statistics. As a result of overlap between the various FSSs (
Table 1), the sample was divided into three groups on the basis of whether participants reported no history of FSSs, a history of one FSS, or a history of two or more FSSs.
Chi-squared tests were used to compare groups on categorical demographic variables of interest. Kruskal-Wallis H tests were used to compare groups on age and time since injury at intake. A linear mixed-effects model (random intercept model) was then used to assess whether a history of FSSs was related to the severity of mTBI symptoms over time, while accounting for other independent variables that could contribute to symptom persistence over time. A history of one FSS or two or more FSSs (with none as the referent), sex (
4), access to compensation at the time of the first visit (
32), history of anxiety or depression (
4), and time since injury were included in the model as fixed effects, because these are established predictors of mTBI outcome. Participant ID was included in the model as a random effect. A second model was then run without including a history of anxiety or depression as a fixed factor to rule out multicollinearity, because many participants with a history of FSSs also had prior depression or anxiety (
Table 1). A third unadjusted model with only the FSS variables as predictors and no covariates was also run. Satterthwaite’s approximation was used to determine p values.
To supplement the primary analysis, where RPQ score was treated as a continuous variable, we also constructed contingency tables to examine the association between FSSs and risk of severe mTBI symptoms at each time point. An RPQ score ≥33 was considered “severe” based on prior research demonstrating that <5% of patients with an mTBI obtain a score this high (
33). Odds ratios were calculated to determine the odds of reporting severe postconcussion symptoms based on whether the participant reported a previous diagnosis of an FSS. Odds ratios were calculated by dividing the proportion of individuals with one or more FSSs and two or more FSSs (exposed group) who reported severe postconcussion symptoms by the proportion of individuals with no FSS diagnosis (comparison group) who reported severe symptoms. An odds ratio of 1.0 represented equal risk in the exposed and comparison groups of having severe postconcussion symptoms. If the odds ratio was >1.0, individuals in the exposed group had increased odds of reporting severe postconcussion symptoms. If the odds ratio was <1.0, participants in the exposed group had decreased odds of reporting severe postconcussion symptoms. SPSS, version 25, was used for all statistical analyses.
Results
Demographic and injury characteristics of the study participants, as well as their self-reported postconcussion symptoms at intake and at the 1-month and 3-month follow-up visits, are presented in
Table 2. Of the participants, 14.1% had a history of irritable bowel syndrome, 8.5% had a history of chronic fatigue syndrome, and 8.5% had a history of fibromyalgia. Additionally, 12.7% (N=18) had a history of one FSS, and 7.7% (N=11) had a history of two or more FSSs. Overlap between histories of FSSs and mental health problems is summarized in
Table 1.
Participants with no FSSs, one FSS, or two or more FSSs did not differ on age (H=3.46, p=0.18) or time since injury (H=2.00, p=0.37). Chi-squared tests showed no significant difference between the three groups on sex (χ 2=0.90, df=2, p=0.63) and access to compensation at intake (χ 2=2.23, df=2, p=0.31) but did show significant difference between groups on history of anxiety or depression (χ 2=8.66, df=2, p=0.01). Of those who had two or more FSSs, 81.8% (N=9) also reported a history of anxiety or depression compared with 38.9% (N=7) of participants with one FSS and 36.4% (N=41) of those with no FSSs.
Results of linear mixed-effects models are presented in
Table 3. Patients with a history of one FSS and two or more FSSs did not differ on RPQ scores over time compared with those with no history of FSSs. Running the model without including history of anxiety or depression as a fixed factor and without any additional covariates did not affect this finding (
Table 3).
A history of one or more FSSs (versus no FSSs) was not associated with increased odds of severe postconcussion symptoms at clinic intake (odds ratio=0.88, 95% CI=0.38–2.03) or at the 1-month follow-up visit (0.57, 95% CI=0.22–1.45) or 3-month follow-up visit (odds ratio=0.97, 95% CI=0.36–2.63). Similarly, statistically significant differences were not found for odds of reporting severe postconcussion symptoms between those with a history of two or more FSSs (versus no FSSs) at clinic intake (odds ratio=1.78, 95% CI=0.45–7.03) or at the 1-month follow-up visit (odds ratio=0.57, 95% CI=0.14–2.37) or 3-month follow-up visit (odds ratio=1.27, 95% CI=0.29–5.65).
Discussion
This is the first study, to our knowledge, to examine the prevalence of FSSs in a sample of adults seen at a specialty clinic for mTBI. Notably, the rates of these FSSs (irritable bowel syndrome, chronic fatigue syndrome, and fibromyalgia) in this patient sample were much higher than the estimates of the rates of these conditions in the general population (
28,
29). Intriguingly, this finding may suggest that common etiological mechanisms (e.g., somatization) contribute to both FSSs and persistent symptoms following concussion. However, there are other plausible explanations. First, the sampling methods in studies assessing FSSs in the general population differed from those in the present study. These studies used large community-based surveys, whereas we surveyed patients who were seeking clinical care (for mTBI). Community-dwelling individuals are likely to report fewer health conditions compared with those surveyed at clinics or hospitals. Second, given that individuals with FSSs report worse health overall (
28) and use health care services at higher rates than those without FSSs (
34), it may be that individuals with FSSs who sustain an mTBI are more likely to seek health care services and to be referred to these specialty clinics.
The second objective of this study was to determine whether a history of FSSs was associated with the postacute course of recovery after mTBI. We found that it was not. Patients with one or more FSSs or two or more FSSs did not have statistically significant higher scores for postconcussion symptoms across time and were not more likely to have more severe postconcussion symptoms at any time point. However, multiple explanations for this unexpected null finding are possible. First, a prior history of FSSs may be truly unimportant for postacute mTBI recovery. This seems unlikely because health status prior to mTBI has been shown to predict mTBI outcome in several previous studies (
4), patients with poor recovery from mTBI report more atypical somatic symptoms than those with good recovery (
17), and FSSs and postconcussion syndrome share common symptom-perpetuating mechanisms, such as anxiety sensitivity (
35,
36) and all-or-nothing coping (
37,
38). Second, FSS history may predict which patients will have a favorable versus unfavorable outcome with mTBI but does not predict differences in symptom severity after patients reach specialty outpatient care. Specifically, patients with a premorbid history of FSSs may be less likely to experience a rapid and complete recovery from mTBI and therefore more likely to seek follow-up care at a specialty clinic (which would explain the overrepresentation of FSS history in the present sample). FSS history may lose its prognostic value at that point. Third, we used self-reported diagnoses of FSSs and therefore did not capture patients who had high somatization and medically unexplained physical symptoms but no diagnosis, patients who were misdiagnosed with another medical disorder, or patients who disagreed with the diagnosis of FSS made by their physician. Using self-reported diagnosis may have limited our ability to detect the impact of premorbid somatization on symptom recovery, which may explain the discordance with previous studies showing that reporting nonspecific somatic symptoms prior to mTBI is related to persistent postconcussion symptoms (
20,
9,
12).
There are several limitations to this study. We recruited patients from specialty mTBI clinics, limiting generalizability to patients with mTBI seen in other care settings. Future research should involve more diverse mTBI samples. Second, we relied on referring physicians to ascertain mTBI cases and did not independently confirm mTBI diagnosis for research purposes. Given that there are many diagnostic criteria for mTBI, which is recognized as a methodological challenge in mTBI research (
39), the criteria used for diagnosis likely varied across physicians. Although independently verifying mTBI diagnosis in future work through a priori defined criteria would not overcome the bias inherent in relying on self-report of mTBI and symptoms at the time of injury, it would ensure consistent inclusion criteria for patients. Third, while our findings on RPQ scores over time were not statistically significant based on FSS history, there was some evidence for a dose-response relationship in our hypothesized direction. Those with two or more FSSs had predicted RPQ scores approximately three points higher, on average over time, than those with no FSSs, whereas those with only one FSS had predicted RPQ scores only approximately one point higher on average over time. Our study was likely insufficiently powered to detect effects of this magnitude. Lastly, our investigation was limited to the most studied FSSs. A more inclusive approach, such as asking about other medically unexplained symptoms, may have increased the ability to find associations between mTBI recovery and FSSs. Future work should implement multiple methods, including self-reported diagnosis, self-reported somatic symptom inventories, and diagnostic interviewing using FSS diagnostic criteria.
It is important to highlight that the etiology of persistent symptoms following mTBI remains poorly understood. Ongoing research has identified several candidate biopsychosocial risk and resiliency factors (
4,
5). In this context, somatization may be an important risk factor for poor mTBI outcome for some individuals. Persistent symptoms after mTBI should only be diagnosed as “functional” (i.e., not fully attributable to neurological disease) for an individual patient on the basis of comprehensive clinical assessment that considers positive diagnostic features, such as atypical or inconsistent symptoms (e.g., word-finding difficulty when speaking to new but not familiar people) and examination findings (e.g., tubular vision defect) (
40,
41).
Conclusions
Treatment-seeking patients with mTBI may have higher rates of preexisting FSSs. However, it remains unclear whether FSS history is an important prognostic factor for long-term outcome, especially within the context of the limitations of this study. Additional research is warranted to investigate the role of somatization in contributing to persistent symptoms after mTBI.