The neurologic examination has two general functions in psychiatry. The first, screening for major neurological disease, is accomplished with an examination emphasizing such “hard” or “major” signs as reflex and motor asymmetry and the Babinski reflex. The results of each component of the screening examination can be described dichotomously as normal or abnormal. These results reflect the presence or absence of neuropathology, particularly that which is focal and acquired later in development. This article deals with the second objective of the psychiatric neurologic exam: evaluating performance decrements in psychiatric patients without identifiable neurologic disorders. This evaluation may be accomplished with an extended exam, which includes assessment for “soft” signs such as inaccurate motor sequencing and bilateral dysgraphesthesia. Such assessments may be described in terms of degree of performance decrement, rather than by the presence or absence of abnormality. These evaluations are often performed by physicians but are also represented in some neuropsychological batteries. Although these two functions—screening and evaluation—are conceptually distinguishable, the examinations serving these ends overlap substantially, and findings with significance in either context are hereafter referred to inclusively as neurologic exam abnormalities (NEA).
Classic descriptions of the psychiatric disorders often included neurological exam findings. Thus, “insane temperament,”
1 “hysteria,”
2 schizophrenia,
3,4 mood disorders,
5,6 and obsessive-compulsive disorder
7 were each thought to have characteristic NEA. By mid-century the American psychiatric literature rarely mentioned neurologic findings. NEA research in child psychiatry then expanded as the concepts of “soft neurological signs”
8 and “minimal brain dysfunction”
9 became popular in the 1960s. This trend led to large pediatric studies
10,11 and to much conceptual and methodologic clarification.
12–15 Studies of NEA in adolescent
16,17 and then adult
18,19 psychiatric patients followed. Studies of NEA in adult psychiatric patients have increased in quantity and quality in recent years; recent texts again include NEA in descriptions of psychopathology.
20,21Since its use by Bender in 1947 to describe findings suggesting possible neurologic disease, the term
soft signs has had other connotations, including a lack of diagnostic or anatomic specificity, a lack of reliability, and evanescence. Also “soft” are the boundaries of the category, considered by some to include such varied features as sinistrality, electroencephalographic dysrhythmias, and learning disabilities, as well as the more widely included NEA.
22 Not surprisingly, some view the topic with derision: “The use of the terms ‘soft signs’ and ‘minimal brain damage’ is diagnostic of soft thinking.”
23 However, summarily dismissing a heterogeneous collection of simple, noninvasive, and inexpensive assessment tools on the above bases could also be regarded as “diagnostic of soft thinking.” First, although anatomic specificity is uniquely valued in neurology, it may not be as important in psychiatry, in which localization is less central to diagnosis. Second, although the terms
hard and
soft imply that the former are reproducible and the latter not, the data suggest otherwise. Third, although some of the “soft” NEA may be evanescent, the extent of this has not been determined in adult psychiatric patients. Because state variation is common in psychiatric syndromes, temporal variability in neurological measures may offer some advantages; variability in performance may itself be an important parameter in psychiatry.
24The term neurological soft signs thus has multiple misleading meanings. Although it may be useful to characterize a set of data as “hard” or “soft” evidence of major neurologic disease, we suggest that the term not be used to describe individual signs.
The potential for the neurologic examination to add to diagnosis, prognosis, and treatment selection in an era of increasing fiscal limitations warrants an examination of the significance of NEA in adult psychiatry. This article, written primarily for researchers, summarizes the current knowledge base on the bedside neurologic examination in adult psychiatry, focusing on methodological issues and on enhancing the clinical relevance of work in this field. Although the boundaries blur between this and such related areas as neurophysiology and neuropsychology, we address techniques that can be readily applied in clinical settings and that would not be considered part of the routine mental status exam.
RELIABILITY AND VALIDITY
Components of the Examination
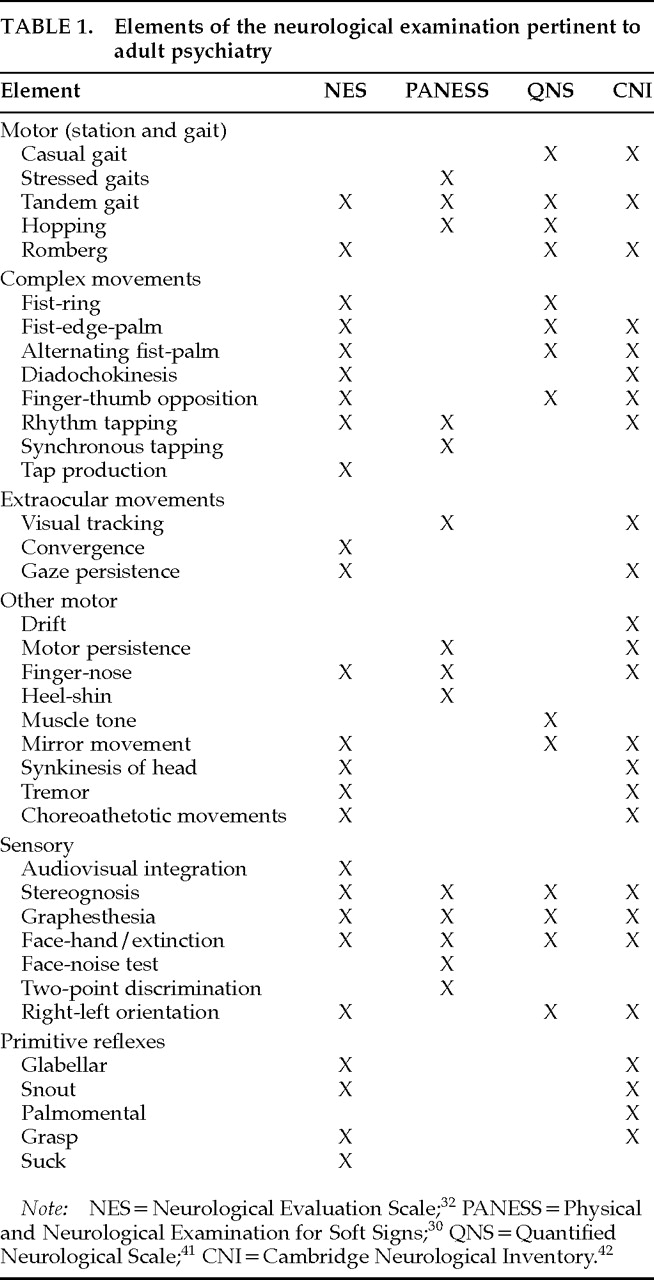
Psychiatric research on NEA has employed collections of neurologic examination items culled from clinical tradition or previous research (
Table 1). Some batteries are partly or entirely drawn from general neurology texts,
25–27 and some are selected from familiar neuropsychological batteries.
28,29 Several scales were developed for use with children in the heyday of research into neurological aspects of pediatric psychiatry
10–12,30,31 and have found application in adult populations.
The Neurological Evaluation Scale (NES),
32 based on a review of NEA in schizophrenia,
33 is the most fully described and widely employed instrument in adult psychiatry.
32,34–39 Convit et al.
40 also developed a composite examination, the Quantified Neurological Scale, which has been used mostly within that group. Chen et al.
41 recently published, for use in adult psychiatry, a heterogeneous inventory of NEA and behavioral observations, with administration guidelines and reliability data for some of the inventory. Some scales
31,40,41 additionally include items usually used to screen for frank neurologic disease but not frequently seen in psychiatric patients. The primitive (release, frontal release) reflexes are often dealt with separately.
42 Rudimentary sensory function, extrapyramidal motor function, spontaneous abnormal movements, and blink rate are not generally included in these neurologic examination schedules. Thus, the available scales tap into somewhat different yet overlapping aspects of neurologic performance. It is important to note this when comparing studies of NEA, particularly when only summary scores are presented.
Each of the instruments referenced in
Table 1 provides directions for administration, as well as some reliability and validity data. These instruments are best viewed as collections of individual examinations that need not be adopted in toto. It may often be more suitable to consider the items individually for inclusion in a clinical or research examination.
Reliability
Numerous threats to the reliability of NEA are worth considering. Many examination items, such as motor overflow, require subjectivity in rating, defying quantification unless special instruments are employed. Interrater agreement is more difficult to achieve when the abnormal response is merely an exaggeration of a normal phenomenon (e.g., tremor and postural sway). Subjectivity can also falsely elevate local agreement (attributable to the shared experience of examiners, as opposed to communicable standards
13) and predispose to drift (weakening of interrater reliability over time
15). Because of the difficulty of directly quantifying some NEA, these studies tend to collapse all data into ordinal or dichotomous formats for uniformity. This practice complicates the statistical approach to reliability assessment of individual exam items, reducing power and producing data that fall in the gray zone between what is suitable for intraclass correlation and what is suitable for the kappa statistic.
43Empirical data nevertheless suggest that interrater reliability is generally quite acceptable.
13 It is compromised primarily in the more subjectively assessed NEA
10,36,44–46 and in those requiring discriminations between normal and slightly abnormal.
10,47 Establishing reliability with some of the “hard” NEA, most strikingly muscle stretch reflexes, may be at least as problematic as it is with psychiatrically significant NEA.
10,44,46,48–50 For example, reliability estimates in the Isle of Wight study
10 were higher for measures of coordination and “developmental abnormalities” than for muscle stretch reflexes. In studies of neurology inpatients, 25%
50 and 43%
49 of routinely assessed NEA fell below the common reliability threshold of kappa=0.4; in two populations of psychiatric patients, the corresponding percentages were 22% and 11%.
36 A clearer understanding of how to conduct the examination improves reliability.
42,44,48 Clinicians might require additional training to assess some of these signs as reliably as in research settings. Such training could be facilitated by video technology.
Less attention has been paid to test-retest reliability. Prospective studies of patients, involving repeated examinations,
28,29,51–53 are required to clarify the state or trait aspects of NEA in psychiatric patients. Interpreting changes in performance over repeated neurologic exams in patients as a function of state change, though, will require data on the stability of NEA in the absence of potentially relevant state changes. Summary indices of NEA are stable in chronic schizophrenia.
32 However, the test-retest reliability of individual NEA has rarely been examined in adult psychiatric populations. Using kappa greater than 0.4 as a threshold, and restricting analysis to items with adequate interrater reliability, two small studies have found that 33%
36 and 44%
54 of NEA could be consistently reproduced. Thus, temporal stability of NEA in adult psychiatric patients remains uncertain. Determining which of the NEA are replicable over time in stable patients is critical for interpreting longitudinal studies of state-related influences on neurologic functioning in psychiatry.
Groupings of Neurologic Exam Elements
Some studies ignore the heterogeneity of NEA, deriving a summary score to represent the overall severity of neurologic dysfunction or the total number of abnormal signs.
26 At the opposite extreme, analyzing NEA individually has limitations due to limited statistical power with ordinal and categorical data, variable reliability among individual items, and problems attendant on multiple tests of significance. Assigning NEA to subscales might allow one to exploit the heterogeneity of NEA while avoiding the limitations of item-by-item analysis.
Some studies have grouped items on the basis of their presumed neuroanatomic substrates.
25,41,55,56 This grouping is debatable in psychiatric patients without focal lesions: NEA do not necessarily have localizing significance in these populations. Rather than anatomical regions, NEA batteries could be indexed in terms of distributed neuroanatomical or neurochemical systems. Others
15,22 categorize individual NEA as signs representing developmental delay, signs consistent with acquired focal brain injury, and subtle variants of focal signs. Many other variables might dictate the association of individual exam items, including their dependence on general intelligence, sustained attention, or motivation.
Five adult psychiatric studies, all involving schizophrenic patients, have used factor analytic techniques to derive item clusters for the neurologic exam,
34,37,51,57,58 with inconsistent results. Further study of the natural organization of NEA in adult psychiatric patients, with adequate numbers of subjects, using appropriate statistics and limiting entered data to reliably assessed NEA, should help to establish defensible subscales.
Relationships With Other Neurologic Tests
A variety of neurophysiologic techniques have been used to characterize phenomena seen in the clinical neurologic exam. Smooth pursuit eye movements (SPEM),
59 visual fixation,
60 and extrapyramidal motor dysfunction
61 have been studied instrumentally, and the palmomental reflex has been quantified following electrical elicitation.
62 These relatively noninvasive and inexpensive methods can obviously help validate the bedside exam, but they have yet to be applied to most NEA. Neurophysiologic methods may also be used to explore biological correlates of NEA; two studies
63,64 found relationships between EEG dysrhythmias and NEA, but others
16,19,65 have not. SPEM dysfunction has been related to NEA among psychotic patients
39,66 and nonpatients.
67 Startle habituation has been related to global neurologic performance.
34Functional neuroimaging parameters seem to be unrelated to NEA in resting patients.
56,68 However, less activation of the appropriate cortical regions is seen during motor tasks in schizophrenia and dementia patients than in comparison subjects.
69–71 Further application of functional imaging and other neurophysiologic methods will be needed to clarify the mechanisms of NEA in patients without acquired focal lesions.
CLINICAL UTILITY
Differential Diagnosis
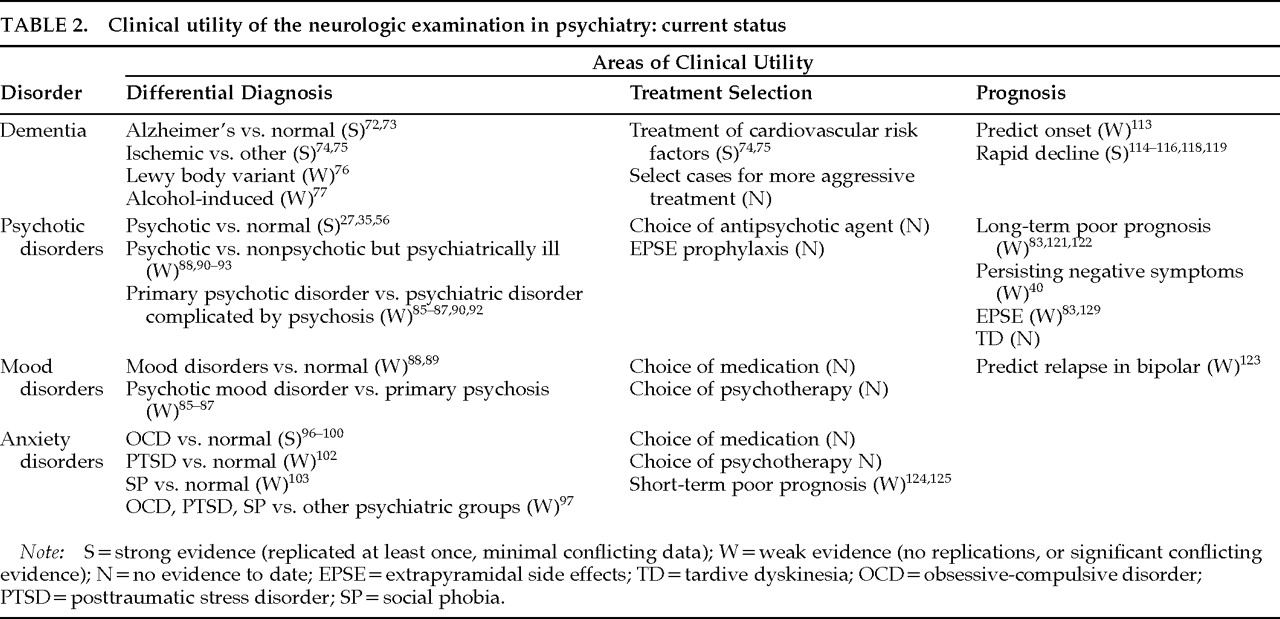
Diagnostic comparisons of NEA have mostly focused on dementia and the psychotic disorders. The neurologic exam could significantly clarify psychiatric differential diagnosis, particularly when the available history is limited. We survey findings to date in dementia and in psychotic, mood, and anxiety disorders (
Table 2, first column).
Dementia has been studied with respect to differential diagnosis and severity. In contrast with normal control subjects, Alzheimer's disease patients have excesses of astereognosis, agraphesthesia, cerebellar findings, olfactory deficits, primitive reflexes, hyperreflexia, abnormal plantar responses, and extrapyramidal findings.
72,73 Focal NEA, gait abnormalities, and dysarthria are associated with multi-infarct dementia, as opposed to other dementias.
74,75 Early extrapyramidal findings may suggest dementia of the Lewy body type.
76 Dementia secondary to alcoholism was strongly associated with ataxia and polyneuropathy in one study.
77 Several other forms of dementia may be related to specific NEA.
78 Primitive reflexes,
72,79 olfactory deficits,
80 and pyramidal,
72 extrapyramidal,
81,82 and other motor abnormalities
79,81 are associated with the cross-sectional severity of dementia; this factor thus needs to be controlled for when comparing NEA across dementias. Potentially, in addition to helping to identify focal and multifocal lesions in patients with dementia, NEA might be found to distinguish between different types of primary degenerative dementia and between dementia and depressive pseudodementia.
The primary psychoses, particularly schizophrenia, are much investigated with respect to NEA (see reviews
33,82). Many NEA, notably abnormalities of motor sequencing, coordination, and higher order sensory function,
33 are more common in schizophrenic than in healthy subjects even when only neuroleptic-naive patients are included.
27,35,56 Parkinsonian findings have also been described in neuroleptic-naive patients.
60,83 Most data suggest that schizophrenic patients have more neurologic dysfunction than nonpsychotic patients, but the evidence is more mixed when comparing psychotic patients with different diagnoses.
33,84–87 Some longitudinal data suggest an improvement in neurological performance with improvement in clinical state and/or treatment with antipsychotic medication.
51,53 Variation in NEA due to clinical state or medications may thus obscure comparisons of diagnostic groups. Significant clinical benefits might be realized if NEA could distinguish between psychotic disorders, but further studies comparing diagnoses while controlling for treatment and clinical status will first be necessary.
Mood disorders have been neglected in NEA research. Abnormalities exceeding those of normal comparison subjects have been found in manic
88 and mixed manic and depressed patients.
89 Differences were found in motor control and sequencing, stereognosis, and graphesthesia. In studies comparing mood-disordered with schizophrenic patients, global NEA were fewer and/or milder in mood disorders,
18,90–92 although two studies
88,93 found few differences. Consistent with early descriptions of melancholia suggesting extrapyramidal dysfunction,
5,6 depression and extrapyramidal dysfunction are related in Alzheimer's disease.
81,94 There are few differences in NEA between unipolar and bipolar patients.
25,29,89,92 The prevalence and diagnostic specificity of NEA in mood disorders remain unclear. The strong effects of psychiatric state on general functioning in the mood disorders, along with the mood-related variability of neurologic functioning evidenced in psychiatric patients,
29,52 suggest that phase of illness will need to be considered. Diurnal variation may also have a significant impact on neurologic function in the mood disorders.
95Anxiety disorders have scarcely been investigated, with the exception of obsessive-compulsive disorder (OCD). NEA are more frequent in OCD patients than in normal comparison subjects,
96–99 although this finding was not replicated in female OCD patients.
100 Specific findings include poor motor coordination,
97,98 disinhibited motor activity,
96,98,99 impaired balance,
97 an excess of left-sided dysfunction,
96,98,99 and extrapyramidal motor findings.
7,101 Difficulties with motor control and with presumably right-hemisphere tasks are fairly consistent findings in this body of work. The diagnostic specificity of these findings to OCD remains unclear.
97 More NEA are seen in posttraumatic stress disorder patients
102 than in control subjects, with significantly greater abnormality in motor sequencing and the palmomental reflex. Social phobia patients had marginally more NEA than normal control subjects in a small study.
103 Further work is needed to address the specificity of these findings to anxiety disorders generally and to specific anxiety disorders, while considering the possible effects of state anxiety and medication.
Treatment Selection and Prognosis
To the extent that they are static or trait-like, NEA might be expected to predict such aspects of psychopathology as illness onset, outcome, and complications of treatment. These are areas in which the neurologic exam has clear potential for complementing clinical assessment of psychiatric patients. These areas of clinical utility are represented in the last two columns of
Table 2.
Onset Prediction:
Follow-up of subsamples from the National Collaborative Perinatal Project found that NEA at age seven, particularly motor abnormalities, predict depression,
14,104 anxiety,
14,104 and delinquency
105 in adolescence and also predict criminality
105 and perhaps anxiety
106 in adulthood. Motor dysfunction and general neurological impairment in preadolescence predicted psychiatric morbidity in the New York schizophrenia high-risk project.
107 Children with motor impairments are more likely to develop schizophrenia by adulthood,
108–110 and sensory deficits may also enhance vulnerability to psychosis in youth
111 as well as in old age.
112 In elderly persons, extrapyramidal findings may predict the onset of dementia.
113Outcome Prediction:
Neurologic findings with possible prognostic value in Alzheimer's disease include extrapyramidal signs,
114–116 myoclonus,
114,117 parietal signs,
114,117–119 and primitive reflexes.
115 In schizophrenia and mood disorders, NEA at baseline may not predict short-term amelioration of psychiatric symptoms.
33,53,89,120 However, negative symptoms persisting after treatment with conventional antipsychotic medication were predicted by baseline NEA.
40 Long-term global outcome in schizophrenia was also anticipated by NEA, including motor sequencing and extrapyramidal dysfunction.
83,121,122 Stable, medicated bipolar patients with neurologic findings were more likely to relapse during follow-up.
123 NEA predicted a poor response to medication in one of two studies of obsessive-compulsive disorder.
124,125 Thus, neurologic signs may have prognostic importance in a variety of psychiatric contexts, with implications for treatment selection.
Predictions of Treatment Complications:
Although NEA might predict neurologic side effects from a variety of psychiatric medications, the only data known to us pertain to typical antipsychotic agents. Tardive dyskinesia, noted to coincide with motor and sensory findings and primitive reflexes,
126–128 may also be predicted by such findings: withdrawal-emergent dyskinesia was predicted by the number of NEA in one study.
26 Low blink rate
129 and other parkinsonian signs
83 predict parkinsonism after treatment with conventional antipsychotic drugs. Baseline neurological data may help to inform choices regarding antipsychotics and prophylactic antiparkinsonian agents.
CONCLUSIONS
Psychiatry is progressively informed by the brain sciences, but it is constrained in applying this knowledge to the clinical assessment of the individual patient by the high cost of the technologies applied in neuropsychiatric research. The neurologic examination is inexpensive and available to all physicians, and thus it has the potential to bridge the gulf between neurobiologic research and clinical practice. It has shown promise as an aid in differential diagnosis, prognosis, and treatment selection in a variety of psychiatric conditions.
The neurologic examination also has important limitations. It offers only indirect reflections of the salient properties of the brain, and it is presumably inferior to such approaches as functional imaging and histopathology in characterizing specific neural systems or in elucidating the neurobiologic bases of psychiatric disorders. Some may thus find it less intellectually exciting than many other techniques, and its utility may lie in more immediately clinical questions. A second limitation is that an extended neurologic exam requires time to complete. Clinicians tend to avoid devoting time to evaluative activities that shed only ambiguous light on the case at hand and thus may be reluctant to perform entire exam schedules routinely. Any extension beyond the traditional cursory “rule out” examination will be adopted only to the extent that it has clear implications for patient care. The limitations on reliability, although neither trivial nor entirely determined (especially in the case of test-retest reliability), appear to be comparable to those of the conventional neurologic exam.
Establishing efficient applications of the neurologic exam for specific clinical questions will require considerable refinement of the knowledge base. As
Table 2 illustrates, many potential clinical applications have been investigated insufficiently, if at all.
First, it must be clearly established, for a given clinical population, which examination items are frequently enough abnormal to be of interest and can be readily assessed with adequate interrater reliability. Batteries must be developed that are limited to such items.
The reliability and validity of individual and aggregate neurologic tests should be assessed as methodically as are neuropsychological tests. These tests will require further validation against other measures, particularly those measuring regional brain function, so that we may better understand the nature of the abnormalities and explore the possibility that these tests offer more efficient alternative routes to similar information yielded by these less accessible methods.
We need comprehensive developmental and normative assessments of such examinations, especially before they are otherwise used in studies involving elderly subjects. The exams should be applied to large samples of diagnostically heterogeneous patients to clarify their value in differential diagnosis; to longitudinal studies to clarify their value in prognosis; and to therapeutic trials to clarify their value in treatment selection. The current tendency toward descriptive studies will have to give way to the testing of more specific hypotheses.
Much of the promise of the extended neurological examination lies in its heterogeneity. Its numerous elements individually have potential utility that may well be lost when they are combined into summary indices of “soft signs” or “neuromotor dysfunction.” Their potential may be realized only through item-by-item analyses. This approach is more feasible if these measures are continuous, rather than categorical or ordinal. Combinations of items should be empirically rather than intuitively determined; this might be done through a quantitative distillation such as factor analysis.
Bringing neurobiological knowledge to bear on clinical psychiatry is a task that can differ conceptually and methodologically from the more vigorously pursued task of uncovering the neurobiological bases of psychiatric disorders.
130 If pursued with comparable fervor to that aroused by the more fundamental questions, such work could have a substantial impact on the practice of clinical psychiatry. The neurological examination offers promising avenues for the reintegration of neurobiology into psychiatric assessment.
ACKNOWLEDGMENTS
The authors thank Joseph Pierri, M.D., for his comments on this manuscript, Melissa Knox for library assistance, and Tamera McLaughlin for secretarial support. This work was supported in part by National Institute of Mental Health Grants MH45203, MH01180, and MH45156 (M.S.K.) and was previously presented at the Society of Biological Psychiatry annual meeting, San Diego, CA, May 15–18, 1997.