Lewy body disease (LBD) is the second most common pathologic correlate of dementia in the elderly, following Alzheimer's disease (AD).
1 Neuropathologic findings in LBD patients usually include those of both typical AD and Parkinson's disease (PD). More rarely, LBD patients may present only with Lewy bodies in the brainstem and cortex, without any Alzheimer's pathology. These patients have been referred to as “pure” LBD patients. In the past, there has been no concordance among neuropathologists on criteria for diagnosing LBD. Relatively recently, consensus guidelines for the neuropathologic diagnosis of LBD have been proposed,
2 although these have yet to be validated across patient populations. It is the co-occurrence of these two different pathologies that complicates both neuropathologic and clinical diagnosis of LBD.
The most commonly reported clinical symptoms associated with LBD are progressive dementia, extrapyramidal signs, visual hallucinations and delusions, and cognitive fluctuations.
3 Other associated features include sensitivity to antipsychotic drugs, orthostatic hypotension, transient clouding of consciousness, syncope, and falling. The similarity of neuropathologic and clinical symptoms of LBD to those of AD and PD has led to an overlapping range of terms being used to describe these patients. Among these are the LB variant of AD, senile dementia of the LB type, diffuse LB disease, and PD in AD.
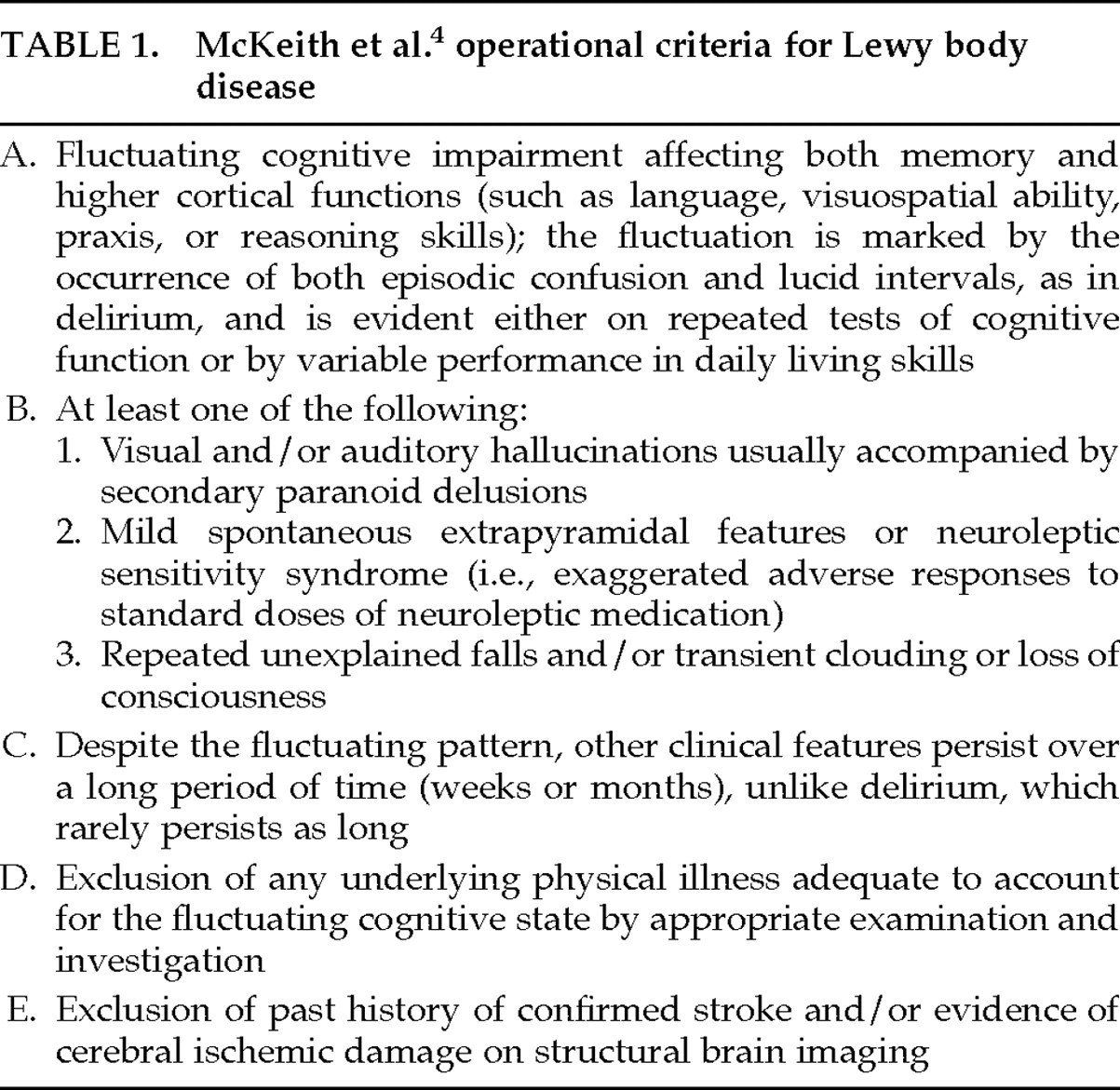
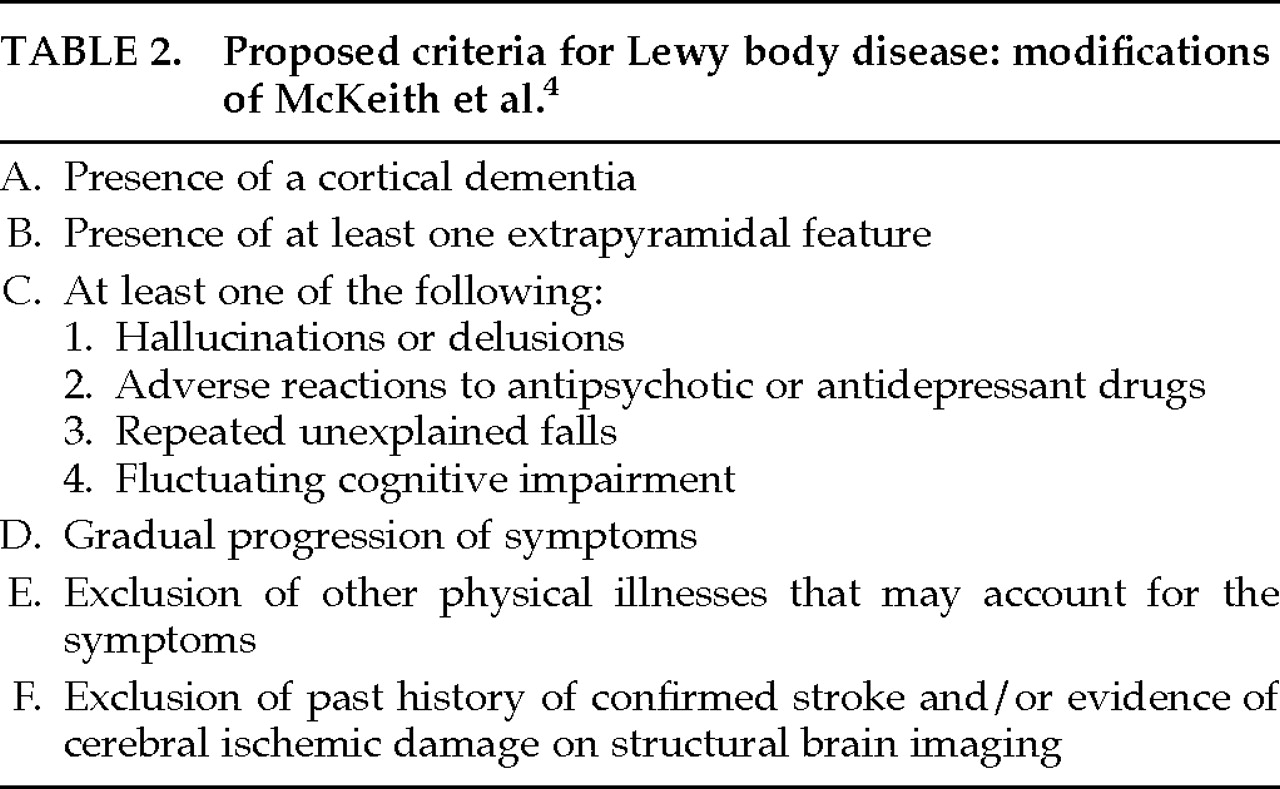
Several sets of criteria for the clinical diagnosis of LBD have been proposed. Among the most influential were the criteria set forth by McKeith et al. in 1992.
4 These criteria, shown in
Table 1, are based on a retrospective study of 21 autopsy-determined LBD cases. Applied to one sample of LBD and AD patients,
4 these criteria, on retrospective review, identified 71% of LBD cases on initial presentation and 86% between presentation and death. Cross-validation of these criteria by the same researchers in a sample of LBD, AD, and multi-infarct dementia (MID) patients yielded a sensitivity rate of 74% and a specificity of 95%.
5 In this latter study, it was reported that less experienced clinicians were more likely to misdiagnose LBD as AD or MID because of a failure to recognize cognitive fluctuations unique to LBD.
In our attempt to use the McKeith et al. 1992
4 criteria with patients evaluated in the University of Rochester Alzheimer's Disease Center (ADRC), we also found these cognitive fluctuations difficult to detect in clinical practice. Furthermore, we hypothesized that all patients with LBD would have at least some extrapyramidal signs, since these patients evidence subcortical LBs at autopsy. The importance of extrapyramidal signs in making a clinical diagnosis of LBD has been supported by Mega et al.,
6 who found that cogwheeling, rigidity, and bradykinesia were 3 of 6 symptoms that helped to distinguish LBD from AD patients. Hence, we adopted a modified version of the McKeith et al. 1992
4 criteria, shown in
Table 2. This modification required the presence of at least one extrapyramidal sign sometime during the early to moderate course of the disease. We also considered that depression, reported to be more prevalent in LBD than AD patients in at least one study,
7 might distinguish LBD from AD patients in this sample. Therefore, we recorded this variable separately.
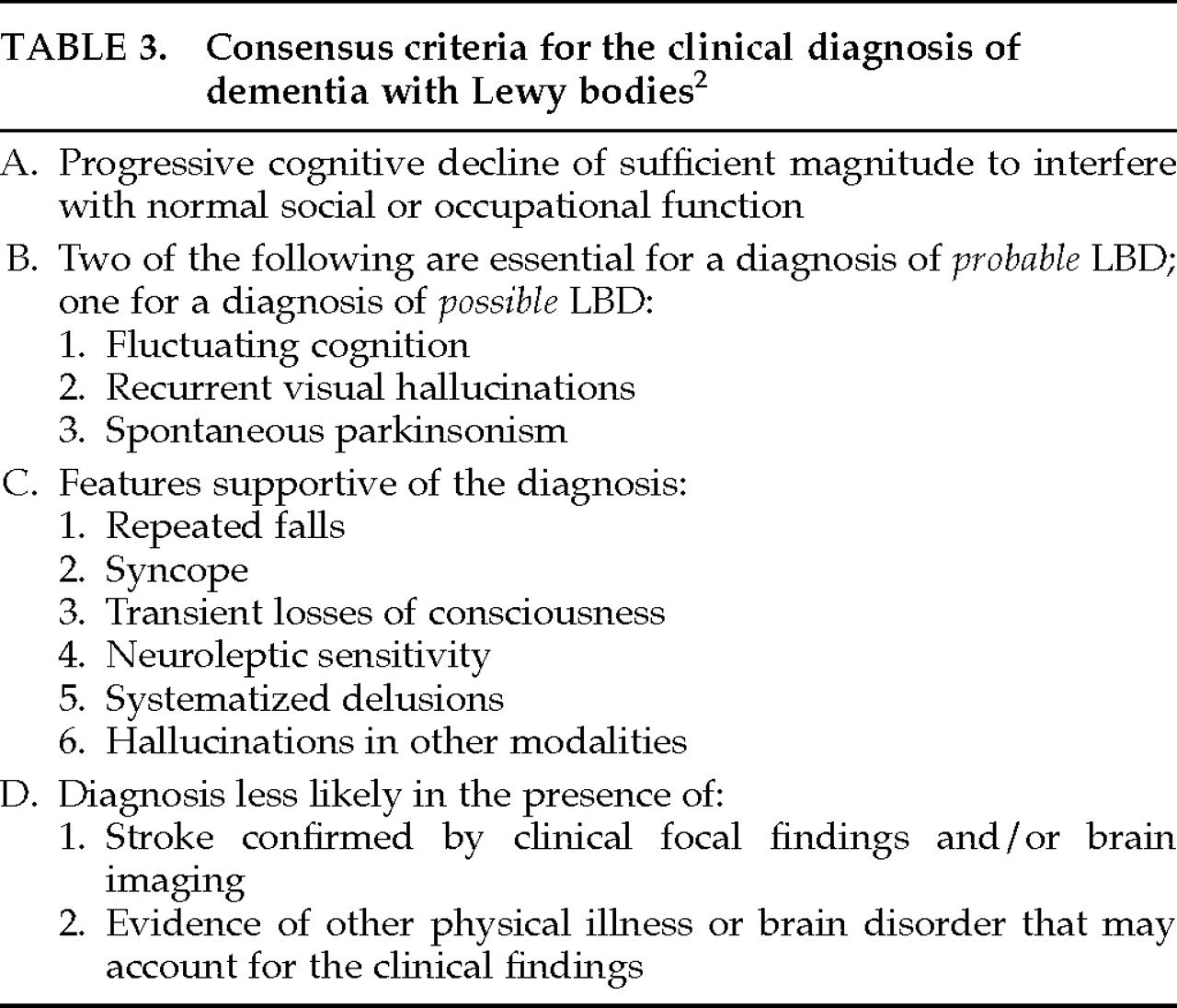
The most recent set of operational criteria for LBD,
2 largely based on the McKeith et al. 1992
4 criteria, was developed during the 1996 proceedings of the First International Workshop of the Consortium on Dementia with Lewy Bodies. The report from this consortium acknowledges the difficulty of assessing fluctuating cognition; the consensus criteria for the clinical diagnosis of LBD (termed
dementia with Lewy bodies) include this as a core, but not essential, feature of the disease.
2 The consensus criteria for making a diagnosis for “probable” and “possible” LBD are outlined in
Table 3.
These consensus criteria were applied retrospectively by Mega et al.
6 to 24 patients who had received premortem diagnoses of AD, PD, or progressive supranuclear palsy (PSP). The sensitivity and specificity of these criteria were 75% and 79%, respectively, for cases found to have “many LBs” at autopsy (4 or more LBs in at least 3 cortical 250× fields). Equal sensitivity (75%) and 100% specificity were attained in this sample when the presence of hallucinations, cogwheeling, rigidity, bradykinesia, neuroleptic sensitivity, and cognitive fluctuations were used as clinical criteria for LBD.
The purpose of the present study was to assess the accuracy of the McKeith et al. 1992 criteria,
4 the consensus criteria,
2 and our own proposed criteria in detecting autopsy-confirmed LB pathology. Two clinicians (M.P., R.S.), blinded to autopsy diagnosis, determined by agreement whether each of 46 patients met specified LBD criteria using the Rochester ADRC database and traditional medical charts. Neuropathologic diagnosis was made by one investigator (A.R.) using published guidelines.
2,8 The goal was to determine the utility of consensus criteria and the presence of extrapyramidal signs in accurate clinical diagnosis of LBD.
METHODS
Subjects
All patients listed in the 1992–1995 Rochester ADRC Neuropathology Database with a neuropathologic diagnosis of LBD (not excluding concomitant AD pathology) were selected as subjects (n=23). For each LBD subject, a patient with the neuropathologic diagnosis of AD was selected from the same database on the basis of temporal proximity to the time of death of selected LBD cases. For both subject groups, only patients without other significant neuropathology (e.g., strokes) were included. In total, the initial sample population consisted of 46 subjects, all of whom had given written consent to participation and enrollment in the Rochester ADRC Neuropathology Study.
Neuropathologic Procedures and Diagnosis
The brain was removed and dissected fresh according to the current protocol of the Rochester ADRC. Among the 24 Formalin-fixed paraffin-embedded sections obtained by this protocol, 10 were examined specifically for this study. Sections included all neocortical lobes, cingulum, anterior and posterior hippocampus with parahippocampal cortex, basal ganglia with insula, and midbrain with substantia nigra. In addition to hematoxylin and eosin (H&E) stains, silver (Yamamoto-Hirano modified Bielchowsky) stain, amyloid (congo red and β-amyloid immunostain), ubiquitin and paired helical filament immunostaining were applied following the standardized biotin streptavidin (ABC) method.
The presence, severity, and topographic extent of AD pathology in both patient groups were determined by using simplified Braak and Braak (B&B) staging criteria.
8 Following the National Institutes of Health recommendation for the diagnosis of AD,
9 we categorized the dementia as “mild” when B&B stages were 1 or 2, “moderate” for B&B stages 3 and 4, and “severe” for B&B stages 5 and 6.
All cases were evaluated for the presence or absence of LBs, following the consensus guideline pathologic criteria.
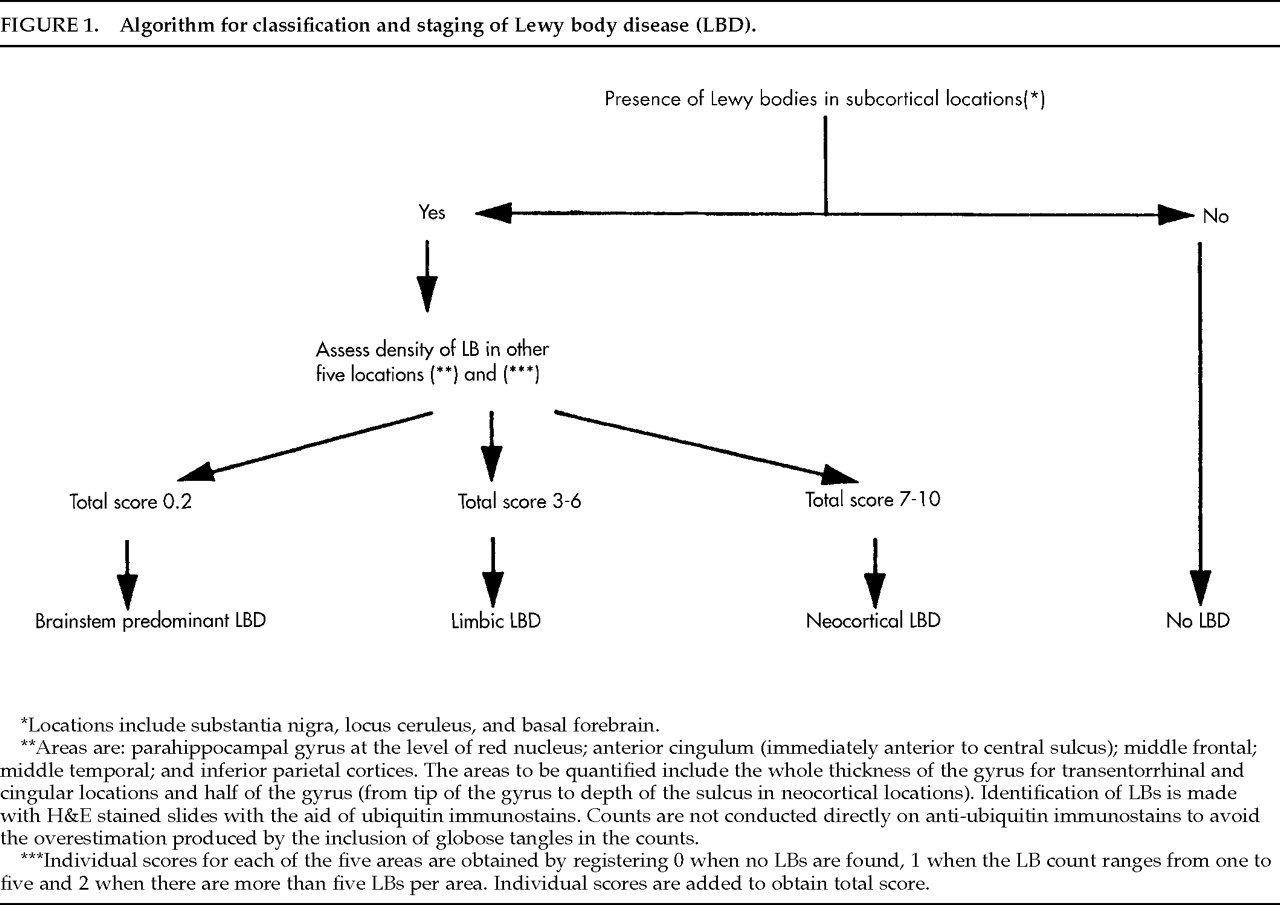
2 Slight modifications of this protocol were made (study of posterior cingulum and superior temporal gyrus instead of anterior cingulum and middle temporal gyrus) on the basis of tissue availability. The presence of LBs was a necessary condition for the LBD diagnosis. The specific algorithm used for evaluating and counting LBs is shown in
Figure 1. These evaluations were conducted on preserved tissue of patients whose autopsies predated the published consensus guidelines for neuropathologic diagnosis of LBD.
Clinical Diagnosis
Two clinicians, one a neuropsychologist (M.P.) and one a neuropsychiatrist (R.S.), both blinded to neuropathologic diagnosis, reviewed the medical history of each subject. Histories were comprised from standardized data forms completed for all subjects enrolled in the Rochester ADRC study and from traditional medical charts maintained by the University of Rochester Medical Center. The ADRC data forms contain variables summarizing demographic and medical information for each patient. Included in these forms are measures of psychiatric features such as hallucinations, delusions, and depression, as well as extrapyramidal features including rigidity, bradykinesia, tremor, and impaired gait. For these items, the presence or absence of a particular symptom is noted along with any relevant descriptors. The forms are completed annually by registered, Master's-level nurses with specialized training and experience in geriatrics. Completion of these forms is based on information collected by ADRC nurses, including patient evaluation, interviews with the patient's family members, and past medical records. Assessments are made according to a standardized protocol developed by geriatric neurologists, neuropsychologists, and neurologists in the ADRC. To help ascertain accuracy of clinical assessments, all completed forms are reviewed by the Research Nurse Coordinator and then by the Data Coordinator to ensure completeness and consistency.
Explicit documentation in clinical notes or endorsement on the structured database forms was necessary for a symptom to be considered present. Although not a variable on any set of criteria, the presence or absence of depressive symptoms sufficient to warrant chart-based diagnosis or to justify the prescription of antidepressant medication was also recorded.
After reviewing the medical history of each subject, it was determined by agreement between both clinical investigators (M.P., R.S.) whether or not each subject met each set of criteria (see
Tables 1–
3).
Statistical Methods
Chi-square and Fisher's exact test were used to compare groups of patients. The predictive power of various clinical variables was assessed by multiple logistic regression with neuropathologic diagnosis (presence vs. absence of LBs) as the dependent variable. In addition to these confirmatory analyses, an exploratory analysis using forward stepwise selection of variables was performed. This stepwise procedure was used because the small sample size precluded an analysis considering all variables simultaneously. The purpose of this exploratory analysis was to identify additional predictors of LBD that could be prospectively test-validated by using different and larger groups of patients.
RESULTS
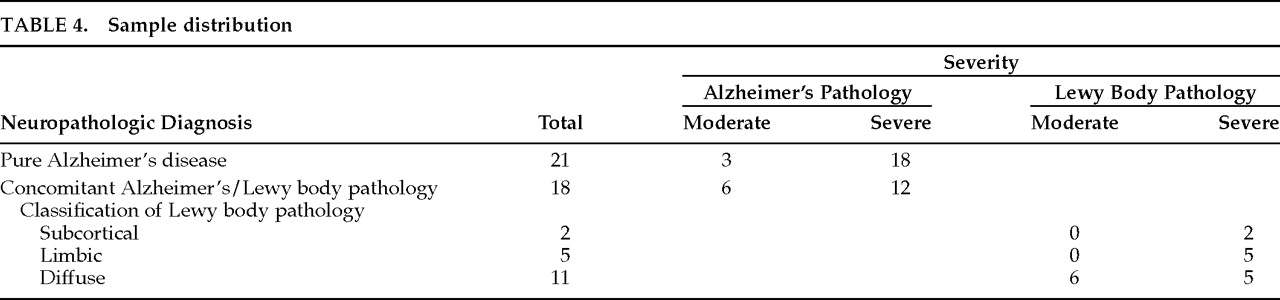
Seven subjects were excluded from the study: 6 had insufficient medical documentation for clinical ratings and 1 had cerebral palsy. Results are based on the data from 39 patients, 21 of whom had autopsy-confirmed AD and 18 of whom had LB pathology with concomitant AD changes. Neuropathologic classification, stage, and severity for each group are outlined in
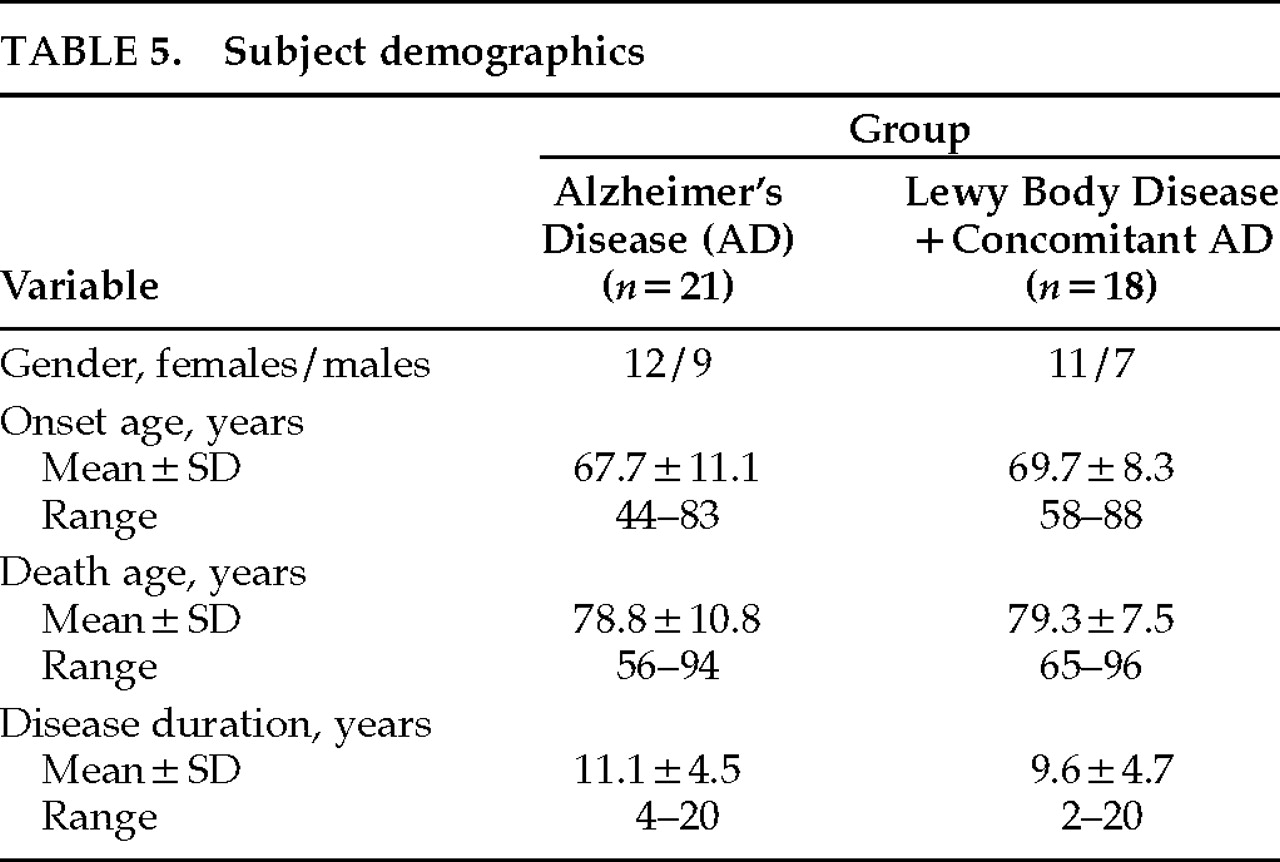
Table 4. Demographics for both groups are shown in
Table 5. There were no significant differences between LBD and pure AD groups on the basis of gender, age at disease onset, age at death, or disease duration. However, 5 of the 21 AD patients (23.8%) had an onset age of younger than 60 years, compared with only 1 of 18 LBD patients (5.6%).
Only 1 subject had LBD listed in our ADRC database as the clinical diagnosis given by a physician who evaluated the patient during life. In this case, LBD was chosen as the secondary diagnosis. This patient met proposed and consensus criteria for LBD, and the diagnosis was confirmed by autopsy. Because the clinical diagnosis of LBD has been recognized only recently, and was added to our database as a diagnostic category early in 1995, the lack of prospective LBD diagnoses is expected. The addition of the LBD diagnostic category actually postdates the time of autopsy for many cases.
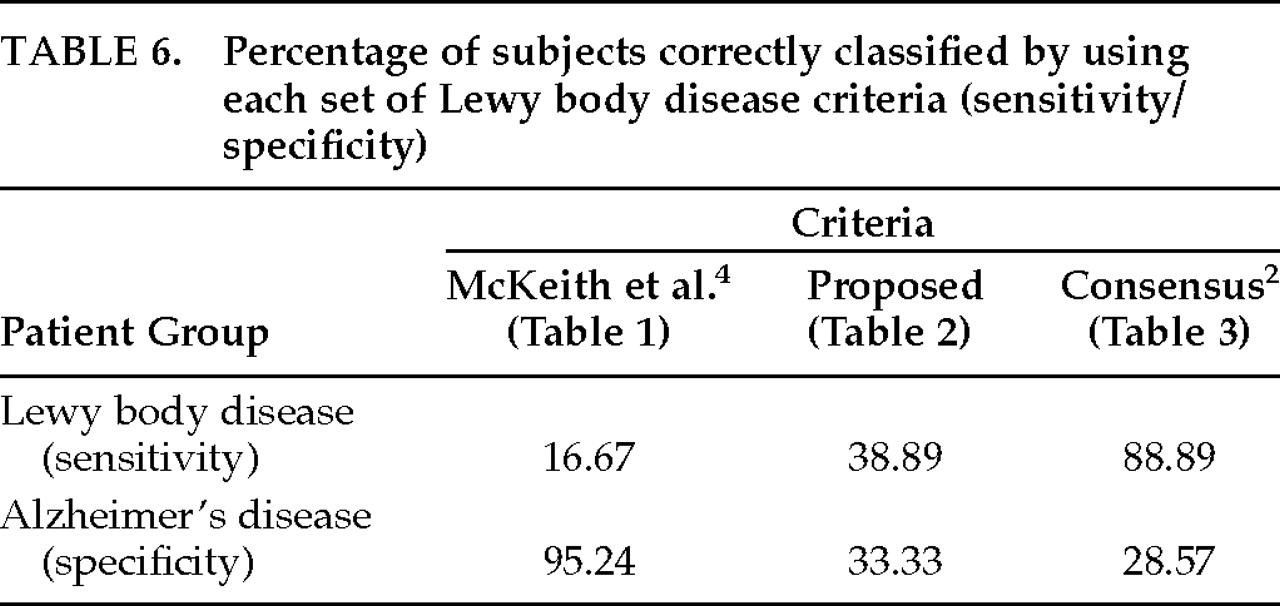
None of the three sets of clinical criteria was accurate in detecting LB pathology. The sensitivity and specificity rates of each set of clinical criteria to LB pathology are summarized in
Table 6. As shown, the McKeith et al. 1992
4 criteria yielded a sensitivity rate of 17% (3/18) and a specificity of 95% (20/21). The high specificity of these criteria is directly related to the high (83%) false negative rate. Fifteen of 18 patients who actually had LB pathology at autopsy did not meet these criteria because there was no evidence of cognitive fluctuations. In fact, only 4 of the 39 patients studied (10.3%) had any evidence of cognitive fluctuations in their medical histories. Three had autopsy-confirmed LBD, and 1 had AD. Because such a small number of subjects met this criterion, which is essential for the diagnosis of LBD in this set, we excluded this set of criteria from the rest of the analyses.
Our requirement of at least one extrapyramidal sign for the diagnosis of LBD did not result in more accurate detection of LB pathology. Instead, our proposed set of criteria fell somewhere between the McKeith et al. 1992
4 and consensus criteria in terms of accuracy, with a sensitivity of 39% and a specificity of 33%. Additional analyses failed to reveal differences between the consideration of early- and late-occurring parkinsonism in the LBD diagnosis. (In those analyses, late-occurring parkinsonism was defined as having presented within the last year of life.)
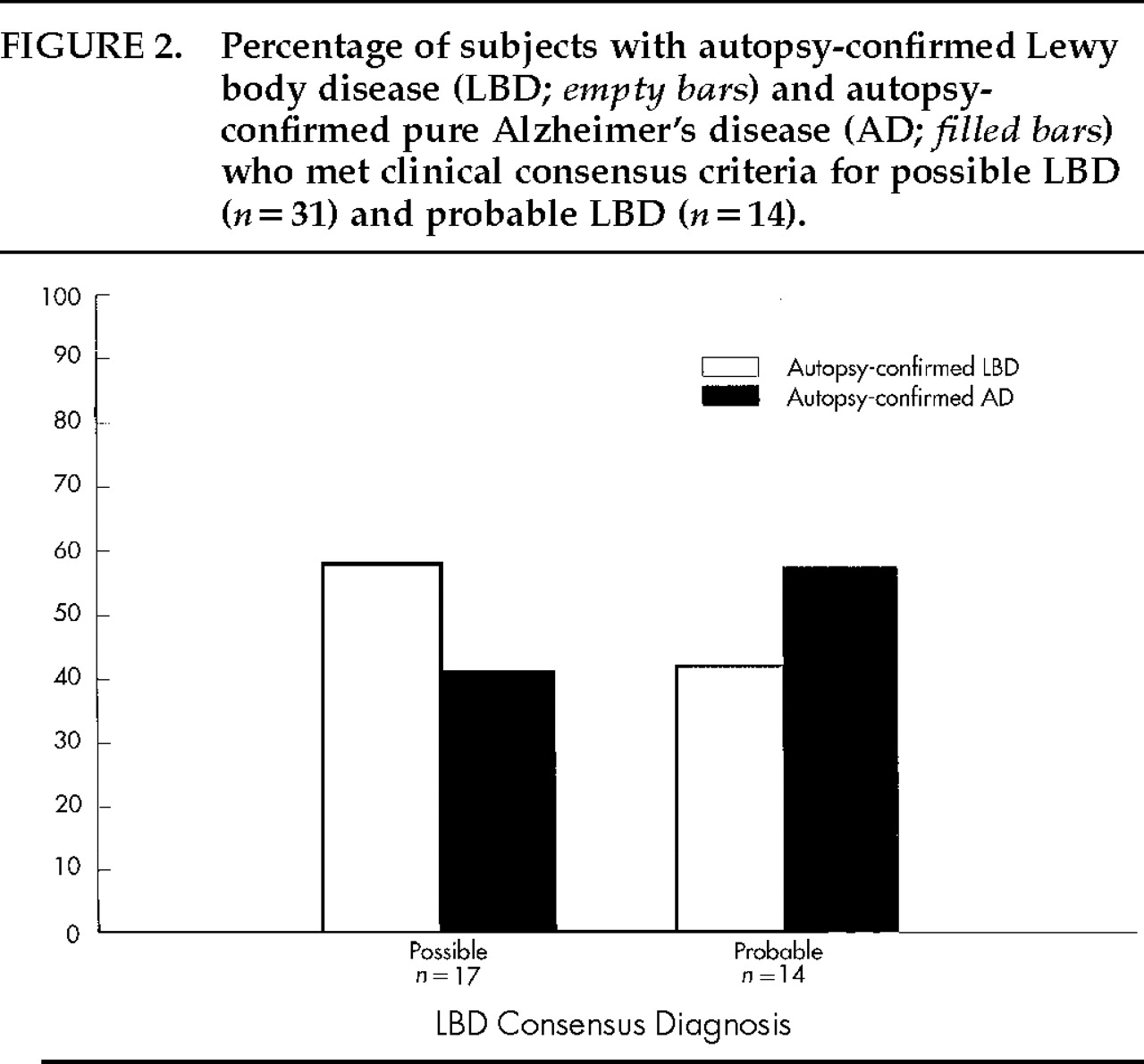
The consensus criteria yielded a high sensitivity (89%) but low specificity (29%) because many AD patients also met these criteria. Therefore, we assessed whether the more conservative diagnosis of “probable” LBD (recommended for research purposes) is more accurate than the more lenient diagnosis of “possible” LBD (recommended for use in clinical practice). Of the 39 subjects studied, 31 (79%) met consensus criteria for possible LBD and 14 (36%) met criteria for probable LBD (in addition to meeting criteria for possible LBD). Among the former group, only 52% (16/31) actually had LB pathology at autopsy, and of the 14 patients who met criteria for probable LBD, only 43% (6/14) had confirmed LBD. Hence, the more conservative diagnosis of probable LBD was not more accurate than a diagnosis of possible LBD in this sample. These results are shown in
Figure 2. Further investigation led to the finding that patients who accurately met clinical criteria for possible LBD but not probable LBD tended to have LB pathology ranging from subcortical (2/10) to limbic (3/10) to neocortical (5/10), whereas patients who correctly met the diagnosis of probable LBD tended to have the most severe form of LBD, present throughout the neocortex. Of the 6 patients who accurately met the diagnosis of probable LBD, 5 (83%) had the neocortical type and 1 (17%) had the limbic type. Including all patients who accurately met clinical criteria for possible LBD, 2/16 (13;pc) had brainstem type, 4/16 (25;pc) had limbic type, and 10/16 (63;pc) had neocortical type.
Since all patients with LB pathology had concomitant AD, the question arose as to whether the severity of concomitant AD affected the accuracy of the clinical diagnosis of LBD. Sixty-seven percent (12/18) of LBD cases had severe versus moderate concomitant AD, compared with 86% (18/21) of pure AD cases. We compared separately the sensitivity of the consensus criteria and the proposed criteria in distinguishing patients with LB pathology from those with pure AD as a function of moderate or severe AD pathology. Although the results of this comparison were not statistically significant, accuracy of LBD diagnosis did seem to improve when concomitant AD pathology was less severe. Using our proposed criteria, there was an 18% increase in sensitivity in detecting LBD when concomitant AD changes were moderate rather than severe. Using consensus criteria, there was a 17% increase in sensitivity and a 6% increase in specificity to LBD when concomitant or pure AD was moderate rather than severe.
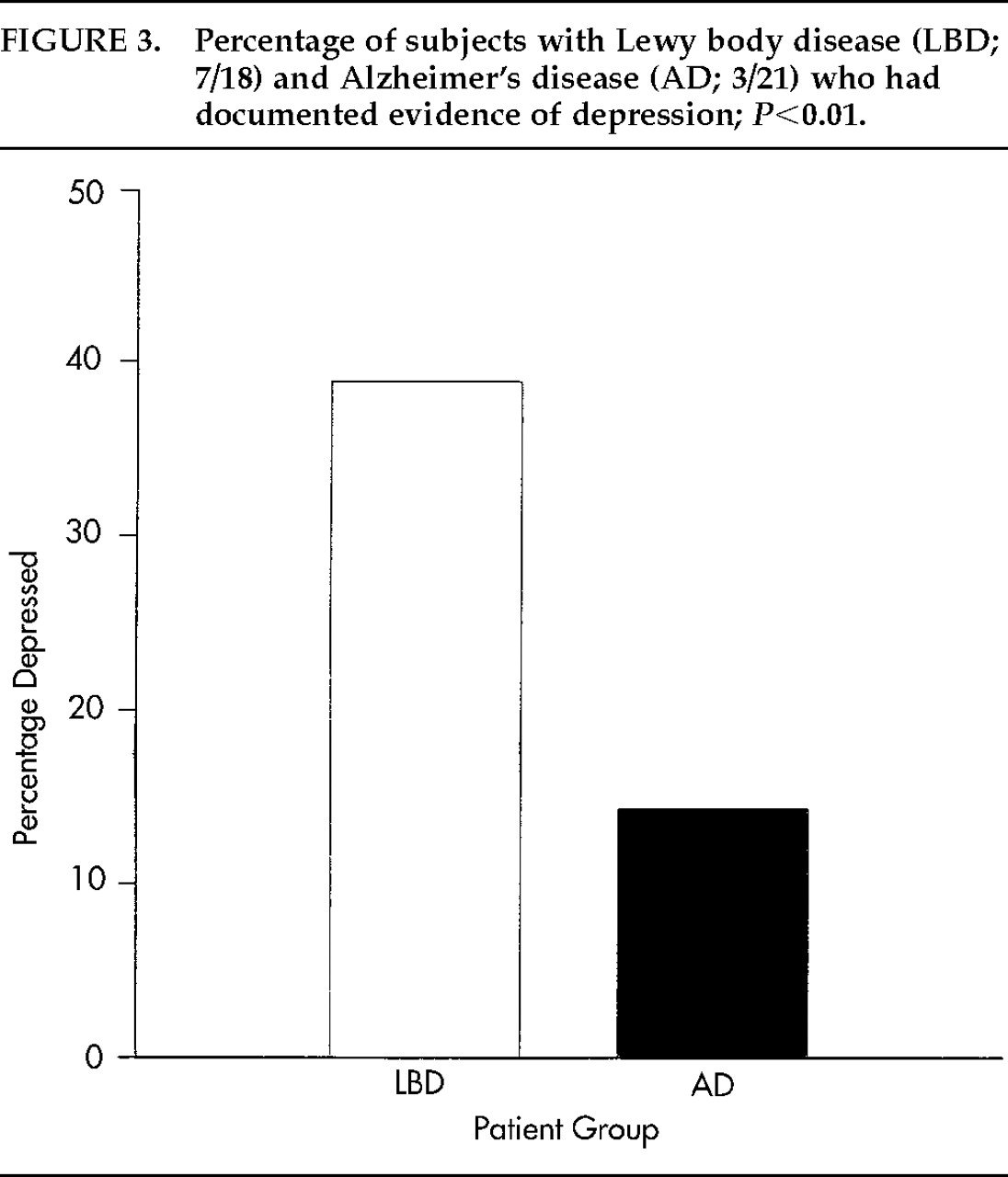
Exploratory logistic regression analyses considering all predictors yielded a model with three variables as predictive of LBD: falls, delusions, and depression. However, of these three variables only depression appeared in the expected direction (i.e., more prevalent in LBD than AD);
P=0.0078, unadjusted for variable selection. As shown in
Figure 3, depression was present in 39% of LBD patients compared with 14% of AD patients, yielding an odds ratio of 16.0 with a 95% confidence interval (1.16, 185.2).
DISCUSSION
Overall, the results of this study fail to confirm sufficient accuracy of any set of clinical criteria tested for predicting LB pathology when it presents with concomitant AD changes. These results generally conflict with those of McKeith and colleagues, who report relative success in distinguishing LBD from AD patients.
2,4,5 Consistent with most other studies, all of the LBD cases in this sample had concomitant AD changes. The accuracy of these clinical criteria in detecting LB pathology in “pure” LBD cases cannot be determined from this study and, to our knowledge, has not been addressed in any published study.
Contrary to our expectations, the presence of extrapyramidal signs did not facilitate more accurate diagnosis of LBD in this sample. This finding differs from that of Mega et al.,
6 who reported improved accuracy in the clinical diagnosis of LBD when extrapyramidal signs were a presenting symptom. That extrapyramidal signs did not occur more frequently in our LBD group compared with our AD group may be explained in several ways. In PD, extrapyramidal symptoms do not usually occur until nearly 80% of substantia nigra neurons are affected. Therefore, brainstem pathology of LBD patients may not always reach a severe enough level to result in clinical signs of parkinsonism. In addition, the substantia nigra of AD patients is grossly affected by neurofibrillary tangles late in the neurodegenerative process,
10 which likely results in clinical parkinsonism.
Consensus guidelines
2 identified a majority of autopsy-confirmed LBD patients in this sample, but they also yielded a high false positive rate, indicating that these criteria are not specific to LBD. The more conservative consensus diagnosis of probable LBD was not more specific to LBD than the more lenient diagnosis of possible LBD. However, patients who accurately met criteria for probable LBD tended to have the most severe form of this neurodegenerative disease. A nonsignificant trend in the data suggested that accurate clinical diagnosis of LBD may be facilitated when concomitant AD pathology is less severe.
In this sample, only 10% (4/39) of patients had any documentation of fluctuating cognition. Hence, the McKeith et al.
4 criteria were not useful in predicting LBD since they necessitate evidence of fluctuations. Other investigators have also reported difficulty in identifying this clinical feature.
5,6 Mega et al.
6 have suggested using a change of 5 or more points on the Mini-Mental State Examination
11 over three administrations within a 6-month period as evidence of fluctuating cognition; however, this objective criterion has yet to be validated.
Exploratory analyses revealed a highly significant association between depression and LBD. In this sample population, the likelihood of having LBD as opposed to AD was 16 times greater if depression was evident. Although preliminary, this finding is noteworthy since depression is not a symptom that is considered as a criterion for LBD in any proposed set of clinical criteria for this disease. Klatka et al.
7 also reported significantly higher depression rates in LBD patients than in AD patients, with frequencies similar to those reported here (50% for LBD vs. 13.8% for AD patients). Also included in that study was a PD control group, in which depression was as common as in LBD. The high likelihood of depression in LBD may reflect a manifestation of LB pathology also common to PD. Although depression has also been reported in AD in varying rates, it is not necessarily considered a constant correlate of the disease. In PD, depression is the most commonly reported mental change.
12 Future investigations of the predictive validity of depression in diagnosing LBD are warranted and should employ standardized measures of depression.
The exploratory analysis also revealed that falls and delusions were more common in AD than LBD patients in this sample. These results were surprising to us, being the opposite of what we had expected. We do not have any solid explanations for these findings. It is possible that our sample was unrepresentative in this regard. Alternatively, it may be that falls and delusions are not good distinguishers of LBD.
There were several limitations to this study, including the use of chart review, retrospective assessment, and operator dependency in clinical documentation and interpretation of information contained within medical charts. In addition, some patients' evaluations were more longitudinal than others, so that some documentations/observations were restricted to specific points in time rather than extended across the later lifespan. This confound is not easily addressed and places universal limitations on investigations of this disease, since most are retrospective in nature. Standardized, prospective, longitudinal documentation by geriatric specialists, including those with expertise in identifying mental, psychiatric, and movement disorders, is critical to further our knowledge of the clinical correlates of this disease. Furthermore, training specialists to identify cognitive fluctuations by some universally established criteria would likely improve the accuracy of LBD diagnosis and the utility of consensus criteria.
An additional limitation to the interpretation of the present results is the forced correlation of longitudinal clinical data with static, end-state neuropathologic data. With these restrictions, it is not possible to ascertain whether AD or LB comes first in the neurodegenerative process and what, if any, relationship exists between the progression of these different pathologies. It is also not clear how each type of neuropathologic change (LBs, neurofibrillary plaques, tangles) relates to behavioral symptomatology, or whether clinical manifestations depend exclusively on the location of neural degeneration. Different groups of investigators have addressed this issue, without consensus.
3 Furthermore, since the neuropathologic diagnosis of LBD has not yet been standardized or validated, clinicopathologic correlations across samples are still difficult to compare.
The number of publications addressing the nosologic entity of LBD has greatly increased over the past few years. Nonetheless, the contention that patients with LBD can be identified clinically with a reliable degree of accuracy is not fully supported by the data. Most of what has been described of this disease has been based on retrospective assessment. To be sure that LBD is a clinically diagnosable disease, prospective, longitudinal studies using proposed clinical criteria would have to be conducted, with subsequent autopsy validation. Results would then have to be cross-validated in other, independent samples. This proposition would also require standardized and validated neuropathologic criteria for making a diagnosis of LBD. Only through such rigor could it truly be ascertained that LBD comprises a distinct, identifiable, age-related neurodegenerative disease process. A further understanding of the clinical profile, pathogenesis, and neuropathology associated with this disease would certainly facilitate our ability to successfully identify and potentially treat this patient population.
ACKNOWLEDGMENTS
This project was supported in part by a grant from the National Institute on Aging, Alzheimer's Disease Center (AG08665), and a Neurobiology of Aging Training Grant (AG00107). This work was presented at a poster session at the 9th annual meeting of the American Neuropsychiatric Association, Honolulu, HI, February 1–3, 1998.