Visual hallucinations are the most common type of hallucination in Alzheimer's disease (AD). They are associated with significant morbidity, including lower cognitive scores,
1,2 more rapid cognitive decline
3,4 behavioral disturbances,
2 and institutionalization.
5A study of visual hallucinations in AD revealed visual hallucinations are significantly associated with disorders of the visual system, including decreased visual acuity and presence of visual agnosia, as well as with older age.
6 These findings support both the role of sensory deprivation and cortical brain pathology in the origin of visual hallucinations. A range of visual abnormalities other than visual hallucinations has been described in AD, including deficits in color discrimination and stereoacuity deficits.
6 Most investigators have suggested that these deficits are caused by neuropathology of visual cortex rather than changes in the optic nerve or retinocalcarine pathways.
7,8 Neuropathology of Brodmann cortical areas 17, 18, and 20 have been proposed as causing these abnormalities;
8 however, the numbers of neurofibrillary tangles and neuritic plaques have been noted to be very low in area 17 (primary visual cortex) but to increase 20–40 fold in areas 18 and 20 (visual association cortex).
9 Considering that Foerster
10 produced complex visual hallucinations by electrically stimulating the visual association area (area 19) but not the primary visual cortex (area 17), we have hypothesized that visual hallucinations in AD may be due in part to neuropathology of visual association cortex.
6A study using single-photon emission computed tomography (SPECT) compared AD patients with and without auditory and visual hallucinations, revealing that hallucinators had relative hypoperfusion of bilateral parietal lobes compared with nonhallucinating patients.
11 It should be noted that this study may simply support prior findings that hallucinations are associated with increased severity of AD and thus with greater hypoperfusion of parietal lobes, rather than localizing hallucinations to this specific brain region. Notably, this study did not look separately at visual hallucinations versus auditory hallucinations, which may well have different neuropathologic correlates. For example, auditory hallucinations, but not visual hallucinations, are strongly and selectively correlated with shrinkage of the left superior temporal gyrus in schizophrenia.
12 For this reason, we believe different types of hallucinations should be examined separately.
In this study, our hypothesis was that AD patients with visual hallucinations would have more neuropathology of occipital cortex than those without visual hallucinations as assessed by volumetric MRI techniques. We predicted that selective occipital atrophy would be significantly associated with presence of visual hallucinations.
METHODS
Consecutive patients attending a university geriatric psychiatry and neurology outpatient clinic who met NINCDS-ADRDA criteria
13 for “probable” AD were asked to participate in the study. The study was explained to patients and to their accompanying family members or guardians. After complete description of the study to the subjects and their family members or guardians, written informed consent was obtained. Patients were first assessed for the presence of visual hallucinations. In this study, as in previous studies of hallucinations in AD, visual hallucinations were defined as present 1) if subjects reported seeing a perception in the absence of an external stimulus or 2) if the caregiver, by history, or the examiner had observed the patient interact by verbalization or behavior with a nonexistent person or object. We applied the following exclusion criteria: 1) if the visual hallucinations occurred only in context of possible delirium (concurrent medical illness, substance abuse, hospitalization, change in medication, recent surgery, or acute change in mental status); and 2) if visual hallucinations had occurred only once in the past month. These exclusion criteria are a combination of those used in prior studies of psychotic symptoms in AD and thus are more rigorous in attempting to exclude visual hallucinations occurring from causes other than AD.
6 Control patients were excluded for any history of visual hallucinations, even if these occurred less frequently than once per month. For each patient with visual hallucinations, a patient without visual hallucinations was matched by cognitive score as assessed by the Mini-Mental State Examination (MMSE)
14 within a two-point range. Matching on cognition was done because it is known that hallucinations in AD are associated with lower cognitive scores.
1,2The subjects' brain MRI scans were acquired by using a MPRAGE pulse sequence
15 to produce T
1-weighted sagittal images. Although two different scanners, a 1.5-T Siemens Magnetom and a 1.5-T Siemens Vision, were used, the images were identical in resolution and had comparable signal-to-noise ratios (SNR). The resulting image matrix dimensions were 256×256×128 voxels with a resolution of 1 mm×1 mm×1.2 mm. Acquisition time for the MPRAGE scan was 9 minutes.
Whole brain volumes were calculated by use of a semiautomated method.
16,17 With this method, a 3-D deformable model of a generalized brain is aligned and stretched to fit onto the brain in the images. The model is then allowed to deform automatically to match the individual shape of the subject's brain. The deformed model was then used to mask out of the images only the voxels within the brain. These voxels were then summed and multiplied by the voxel sizes to estimate the volume of the brain.
Region volume estimates were calculated by an identical volume estimation method except that region surfaces outlining the occipital lobe were manually outlined. Because lateral regions of occipital cortex are more difficult to distinguish reliably, the center 44 sagittal slices were assessed as a measure of occipital atrophy rather than total occipital volume. Two raters (S.H., M.L.S.) were blind as to the patient's hallucinator status. A Pearson correlation of interrater reliability for whole brain volumes revealed Pearson r=0.991, two-tailed P<0.0001, and for occipital volume measures, r=0.75, two-tailed P=0.002.
To control for differences in subjects' brain size, a ratio of measured occipital volume to whole brain volume was calculated and then compared by Student's t-test between hallucinators and nonhallucinators.
RESULTS
Fourteen patients were examined, seven with visual hallucinations matched by cognition to seven without. There were no differences between the two groups in gender, age, or cognitive score (2 males, 5 females per group; mean age=74.2±3.1 years, mean MMSE=19.8±5.2). Patients with visual hallucinations had a mean whole brain volume=2,277.05±503.76 cm3 while nonhallucinators had a mean whole brain volume=1,829.74±272.3 cm3 (t=2.07, df=12, P=0.06). Mean occipital assessed volumes in hallucinators was 39.62±6.19 cm3 as compared with nonhallucinators, 42.58±7.46 cm3 (t=–0.68, df=12, P=0.509). As noted, there were no significant differences between the groups on either variable.
Next the ratio of measured occipital volume to whole brain volumes was compared between hallucinators and nonhallucinators, and this did reveal a significant difference between the two groups. Hallucinators had a ratio of 0.0182±0.005, whereas nonhallucinators' ratio was 0.0235±0.004 (t=–2.37, df=12, two-tailed P=0.035).
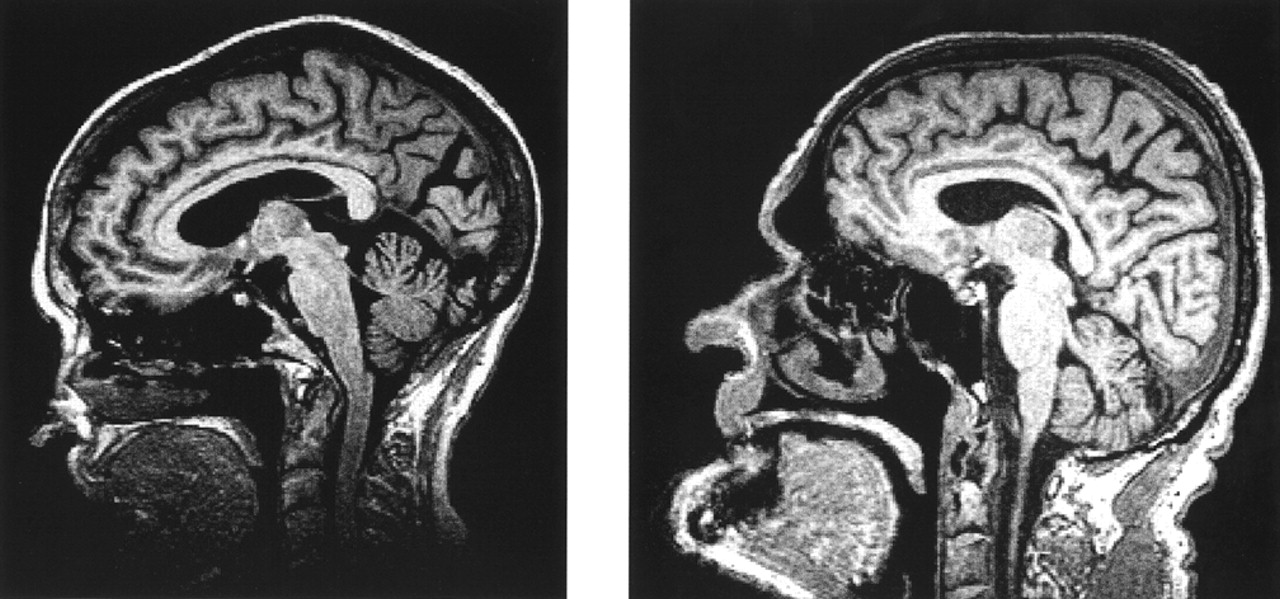
Figure 1 shows an example of two patients matched on cognitive score revealing increased occipital atrophy and widening of the parietal–occipital sulci in the visual hallucinator.
DISCUSSION
In this study, a decreased ratio of measured occipital volume to whole brain volume was associated with visual hallucinations. This result supports our hypothesis that visual hallucinations may be related to neuropathology of the occipital lobe. Ours is the first study examining a specific type of hallucination in AD with attempts to link it with pathology of a specific brain region. Our study supports a growing body of literature linking visual hallucinations to pathology of the visual system. Although occipital atrophy has been examined in this study, it should be noted that visual hallucinations are likely of multifactorial origin. As noted previously, decreased visual acuity and greater age have also been correlated with visual hallucinations in AD.
It should be noted that our results could not be accounted for simply by differences in whole brain volumes between the hallucinating and nonhallucinating groups, but were specific for the occipital region. Limitations to this study include the small sample size, and thus possible subject selection bias, although the small sample size and our results also suggest that this finding may be a strong one.
In summary, our study supports the hypothesis that increased occipital atrophy in AD is associated with visual hallucinations. Further research directions would include replicating this finding as well as examining occipital neuropathology in other disorders with visual hallucinations. It would be of interest to examine visual hallucinations via functional imaging such as SPECT or functional magnetic resonance imaging of occipital lobe in AD patients. This study adds to a growing body of research supporting the association of visual hallucinations with the pathology of the visual system across different disorders.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the National Institute of Mental Health (K07 MH0119901A1).