There have been numerous studies showing a range of brain abnormalities in schizophrenia, specifically ventricular enlargement, reduced brain volume, regionally selective reductions in cortical volume of the temporal lobe (particularly in the amygdalo-hippocampal region) and reduced volumes in prefrontal and parietal cortices, basal ganglia, and diencephalon.
1 What has been less clearly established is the recognition of possible cerebellar correlates in schizophrenia. Studies
2 utilizing different neuroimaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) have been documented. One study revealed CT scan evidence of third ventricle enlargement in association with cerebellar atrophy in 55 young male patients with schizophrenia compared with healthy comparison subjects, thus raising the possibility of a subset of patients with this neuroanatomic correlate.
2 More recent structural MRI studies
3,4 support some of the earlier CT scan findings. Jacobsen et al.
3 examined cerebellar morphology with MRI in 24 patients with childhood-onset schizophrenia and found the volume of the vermis and inferior posterior lobe to be significantly smaller than in comparison subjects. Conversely, Levitt et al.
4 used MRI to measure cerebellar and vermis volume in 15 patients with schizophrenia, finding
increased vermis volume in patients versus comparison subjects, along with increased cerebellar hemispheric volume asymmetry.
Wassink et al.
5 conducted a longitudinal study of 63 subjects with schizophrenia spectrum disorders, examining whether brain morphology, as imaged by MRI early on in the disease, predicted psychosocial or symptomatic outcome. Of the four areas studied (cerebellum, cerebrum, temporal lobes, and ventricles), the cerebellum emerged as the primary brain region that correlated with outcome measures. Biyearly, prospective assessment of patients for an average of 7 years revealed that smaller initial cerebellar volumes predicted a longer duration of negative and psychotic symptoms as well as more psychosocial impairment. At the tissue level, structural abnormalities of the vermis in chronic schizophrenia have been documented in macroscopic and microscopic pathology investigations.
44Functional neuroimaging studies are few, and they reveal mixed results. Using PET scans, Volkow et al.
6 found a lower metabolism in the cerebellum of 18 medicated patients with schizophrenia, compared with comparison subjects. Using PET analysis, decreased cerebellar blood flow was found in 14 subjects with schizophrenia, compared to 13 comparison subjects, while they performed task testing aspects of short- and long-term memory.
7 Eluri et al.
8 examined 12 individuals with schizophrenia for abnormalities in proton magnetic resonance (MR) spectroscopy in the pons and cerebellum. They found significantly lower metabolic spectra in the pons, but not the cerebellum, in those 12 individuals, as compared to comparison subjects.
Another approach, undertaken by Kinney et al.,
9 sought neurologic signs in a proband sample of 54 schizophrenic or schizoaffective patients. This sample had a significantly greater proportion of individuals with signs of cerebellar dysfunction than any of the comparison samples, which included 44 comparison subjects, 24 patients with substance abuse disorder, 37 patients with bipolar disorder, and 73 of the probands’ nonschizophrenic parents and adult siblings.
Rapoport et al.
10 reviewed some of the literature linking the cerebellum to schizophrenia and concluded that, due to weakness such as insufficient replication and lack of comparison groups, the data are to be interpreted with caution. Indeed, it is unclear whether the changes observed are due to cerebellar insult per se or by distance effects to cortical areas that are connected with the cerebellum.
11The Cerebellum and Cognition
In addition to psychiatric illness, the functional role of the cerebellum is being rethought in
normal cognitive processes. In the past, the role of the cerebellum was thought to be limited to gait, posture, motor coordination,
12 and certain aspects of speech.
13 Botez
14,15 described a series of patients with cognitive impairments subsequent to cerebellar insult. Schmahmann
16 reviewed the cerebellar contribution to higher cognition, delineating a number of important projections from the cerebellum to structures of the forebrain, limbic system, hypothalamus and brain stem, as well as multiple thalamic structures. In addition to the role the cerebellum plays in autonomic/vegetative and affective behaviors, aspects of cognition, involving modulation of thought, strategic planning, spatial and temporal parameters, learning, and some components of memory and language also appear to be modulated by this structure. He localizes the majority of these cognitive capacities to the lateral cerebellar lobes. Leiner et al.
17 reviewed functional imaging data, finding trends of cerebellar hemispheric activation in people engaged in language processing and verbal memory tasks. As well, data from simian brain mapping studies show that the cerebellum sends a significant projection of nerve fibers to cognitive areas of the prefrontal cortex.
17Neuropsychological deficits are increasingly being described in patients with cerebellar dysfunction. Schmahmann and Sherman
18 assessed 20 patients with cerebellar pathology and found impairments of executive function, difficulties with spatial cognition, including spatial organization and memory, personality changes and language deficits, coining this presentation “cerebellar cognitive affective syndrome.” While there were some limitations to this study (small sample size, no comparison group), the presentation of “frontal-like” behavioral manifestations is congruent with most other reported deficits in these patients. Indeed, as early as 1985 Botez et al.
19 described transient cognitive dysfunction subsequent to reversible chronic cerebellar ataxia following phenytoin treatment in two epileptic women. Paradiso et al.
20 examined the structure/function relationship between in vivo cerebellar size and higher cognitive function in a sample of healthy young subjects. They used measures of general intelligence, motor dexterity, verbal and visual memory, and covaried these measures with cerebellar size. Cerebellar volume significantly correlated with the ability to retain already encoded information in the verbal domain and also with fine motor dexterity. Chafetz et al.
21 conducted neuropsychological assessments of two patients with acute ischemic strokes who had solitary cerebellar infarcts. Findings suggested lesion-associated deficits in higher aspects of cognition such as visuospatial reasoning, verbal and visual memory, and intellectual and executive functions (see also Leiner et al
22).
The finding of complex neuropsychological deficits in patients with cerebellar insults, either acquired or developmental, such as infarcts,
22 autism,
23 and schizophrenia
24 suggests that, in all, a broadened concept of cerebellar function has emerged as research extends cerebellar processes beyond the motor sphere.
The Cerebellum and Neuropsychiatry
The majority of data in this area comes from single case studies and small case series. Hamilton et al.
25 reviewed case reports of cerebellar disease with psychiatric symptoms, and reported on four cases: two with schizophrenia/schizoaffective disorder and one with bipolar disorder, each with different cerebellar lesions, and a fourth case of psychosis with cerebellar degeneration.
Keddie
26 reported on a family with hereditary ataxia that was complicated by paranoid psychosis. Sandyk
27 reported on a 27-year-old man diagnosed with paranoid schizophrenia who developed
onset of psychotic symptoms after removal of a cerebellar tumor 14 years prior. Jurjus et al.
28 reported on a case of cerebellar degeneration with “schizophrenia-like” psychosis. The latter propose an etiologic link given the chronology of symptoms, the absence of personal or family neuropsychiatric history, and the lack of alcohol use, drug use or trauma.
More recently, Spranger et al.
29 reported on a family with familial hemiplegic migraine and cerebellar ataxia. They suffered recurrent episodes of acute paranoid psychosis, with anxiety and visual hallucinations, that were associated with migraine attacks. Because of evidence linking this disorder to chromosome 19, the authors hypothesize a link to a mutated calcium channel and the alpha 1A voltage-dependent calcium channel gene (CACNL1A4). Tashiro et al.
30 reported on an autopsy case of spinocerebellar ataxia type 6, along with a mild CAG-repeat expansion in the CACNL1A4 gene, in a 52-year-old man with cortical cerebellar atrophy and a diagnosis of schizophrenia. The gene for spinocerebellar ataxia type 1 (SCA1), on chromosome 6, which is also a CAG expansion mutation, is another candidate gene for schizophrenia. Joo et al.
31 found significant associations between allelic frequencies of this gene in 49 patients with schizophrenia compared with comparison subjects.
In fact, nearly 20 diseases over the past decade have been discovered to have expanded trinucleotide repeat sequences (reviewed by Margolis et al
32). This expansion mutation, which increases the length of a region of DNA making it unstable, has been identified in Huntington’s chorea, two forms of the fragile X syndrome, and myotonic dystrophy. These mutations now have a proposed etiologic role in psychotic illness and autism.
To illustrate, we report the case of A.A. who presented with cerebellar signs as well as symptoms of schizophrenia. The information was gathered on chart review.
Presentation of the Case
The patient A.A. is a right-handed 26-year-old man. His perinatal history was unremarkable except for mild toxemia in his mother during pregnancy. His first hospitalization was at 3 years of age after approximately 24 hours of rapid breathing and loss of balance at the end of a week of cold-like symptoms during which he received cold medication and ASA. Difficulties with balance and a delayed onset of walking were reported on history. Tachypnea (50/minute), weakness in the lower extremities, and gait ataxia were noted on physical exam. Blood gas measurement revealed a pattern of respiratory alkalosis. Electroencephalogram was abnormal, with occipital dominant paroxysmal activity, potentially epileptiform, and most pronounced in the left posterior region. The ataxia resolved on day 3 of admission, and blood gases normalized by day 5, with only supportive treatment.
The patient was admitted again at age 7 for unsteadiness of gait, occurring after several days of feeling weak, without appetite, and with a decrease in his activity level. For about 48 hours prior to admission the ataxia worsened, he developed difficulty coordinating his hand movements, his speech became slurred, and he was having some trouble swallowing. Physical exam revealed a normocephalic boy, with a head circumference in the 50th percentile. Speech was slurred and monotonous, and swallowing difficulties were noted. There was no evidence of cranial nerve abnormalities. Mass, tone, power, and sensory exams were normal, and reflexes were symmetrical, with downturned toes bilaterally. There was marked dysmetria in the hands and feet, and severe truncal ataxia both sitting and standing. CT scan showed a large cisterna magna, and EEG revealed sharp irregularities over anterior head regions, perhaps originating in the deep midline structures (
Table 1). A diagnosis of acute cerebellitis, of unknown etiology, was made, and symptoms were almost entirely resolved at his 2-week follow-up.
At 10 years of age a mild bilateral conductive hearing loss was diagnosed with audiologic testing. At age 14 A.A. consulted with orthopedics and was found to have an idiopathic thoracolumbar scoliosis. He also complained of shakiness and general weakness, but his neurological exam was normal.
At age 15 A.A. had his first psychotic episode. He developed paranoid delusions, suicidal ideas, and described somatic hallucinations. There was catatonic posturing at presentation, which resolved with Haloperidol. Blood and CSF studies (
Table 1), along with neurological examination, were normal. Ophthalmologic exam revealed a pale right temporal disc. A.A. left hospital against medical advice after 1 month, still with residual psychotic symptoms.
The patient had his second psychiatric admission almost 5 years later, at age 20, lasting 2½ months. He had taken no medication for about 4½ years before this admission. His family, however, reported emotional instability, reckless driving, and intermittent difficulties working with his father doing industrial cleaning. He had had 1 week of worsening behavior, becoming delusional about having AIDS, and was hearing a voice telling him to kill people. He developed a short-lived catatonic mute state while in the hospital. Intellectual assessment revealed “borderline” intelligence, and he received a diagnosis of schizophrenia, and was discharged on haloperidol.
About 1½ years later another psychotic episode brought A.A. back to hospital. He was found confused and disorganized, thinking he was an undercover police agent, and again had some catatonic posturing. Blood gas testing revealed respiratory alkalosis (
Table 1). While hospitalized, A.A. developed confusion, unstable respirations, and an ataxic gait. Neurologic consultation revealed gait ataxia, dysarthria, a slowing of rapid alternating movements, but no dysmetria or nystagmus. CT scan revealed defined hypodense areas in the cerebellar hemispheres bilaterally, involving both dentate nuclei, along with mega cisterna magna. A repeat CT scan 15 days later was read as having no cerebellar abnormality. Diagnoses of mania and bipolar disorder were made, lithium and Tegretol were started, and antipsychotic medications were discontinued with the idea that they may be related to A.A.’s gait problems.
From age 22 to 27 years, A.A. has had 13 more hospital admissions. Different diagnoses have been suggested, and various pharmacotherapies have been attempted. A transient subclinical hypothyroidism resolved with discontinuation of lithium. Clinical presentation has been similar on many occasions, with paranoid and grandiose delusions, excitement, auditory hallucinations, incoherent or pressured speech, and catatonic and stereotypical movements.
During four of the 13 admissions there were blood tests revealing a low bicarbonate or elevated pH. Clinically, these were described as paroxysmal episodes of “hyperventilation” or “shortness of breath.” Of these four admissions, drowsiness was noted at presentation in two. In one of these, admission followed 4 days of drowsiness, peripheral trembling, ataxia, and dysarthria, but no psychotic symptoms were documented. Medication levels, blood work including creatinine kinase, and temperature were all normal. It was thought to be an EPS-related episode, and the anticholinergic medication was changed.
The patient underwent final admission at the time of this study, which followed a 1-week period of decreased sleep, increased arousal and startle, bizarre and disorganized behavior, and self-talking. At admission he was mildly disorientated, agitated, and had poor concentration, with a fragmented thought form and incoherent rambling. He had marked dysarthria, a moderately wide-based gait, and complained of intermittent dizziness (
Table 1). After 2 weeks A.A.’s mental state had improved considerably, which paralleled improvements in his gait and speech, and the dizziness was no longer present. A marked episodic hyperventilation was notable throughout admission, worsening at times of increased stress. Low serum bicarbonate was found. Neurological assessment revealed slowed fine finger movements, a mild wide-based gait, and mild extrapyramidal features. Familial hemiplegic migraine was hypothesized. However there was no personal or family history of migraine, and A.A. did not complain of headache.
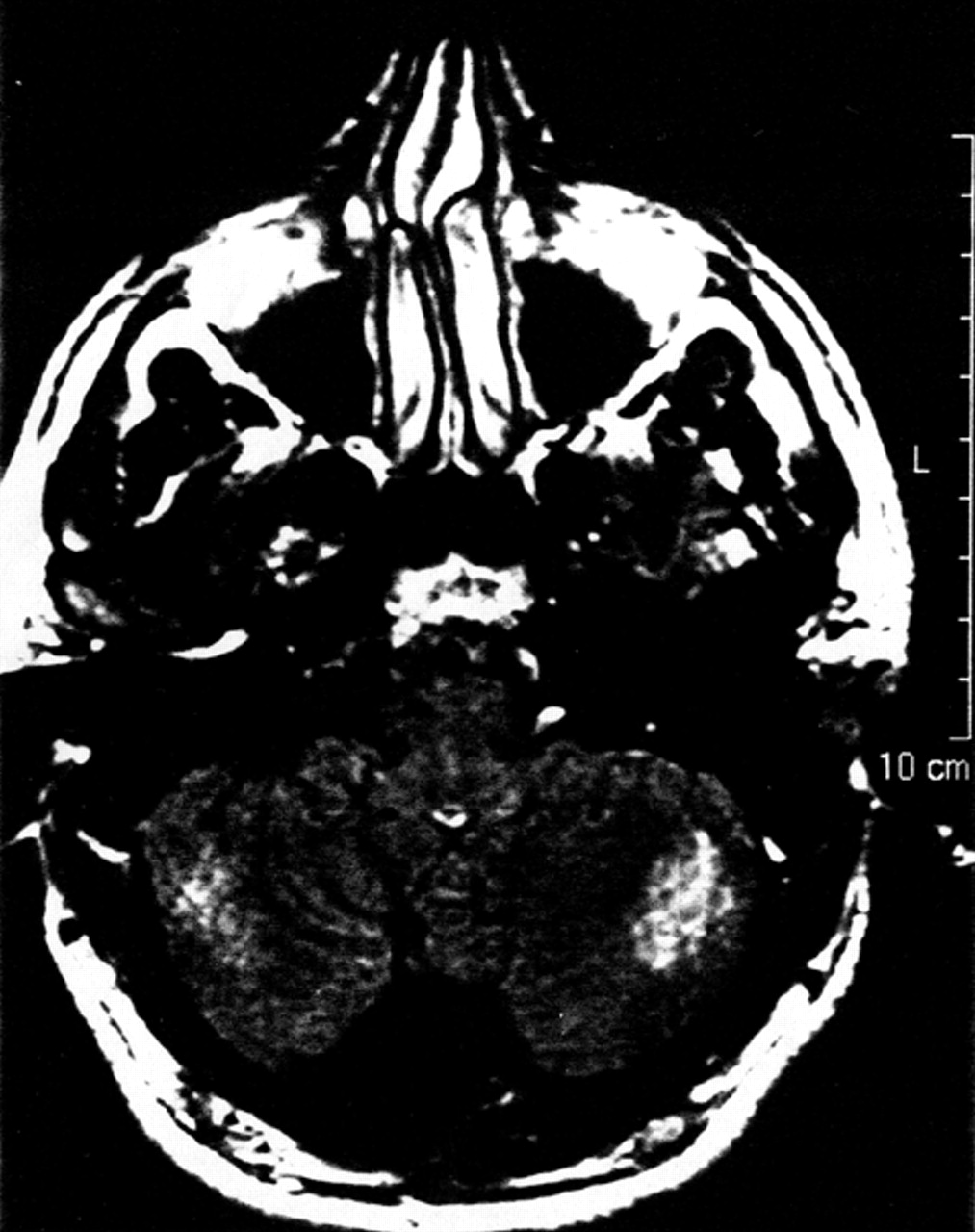
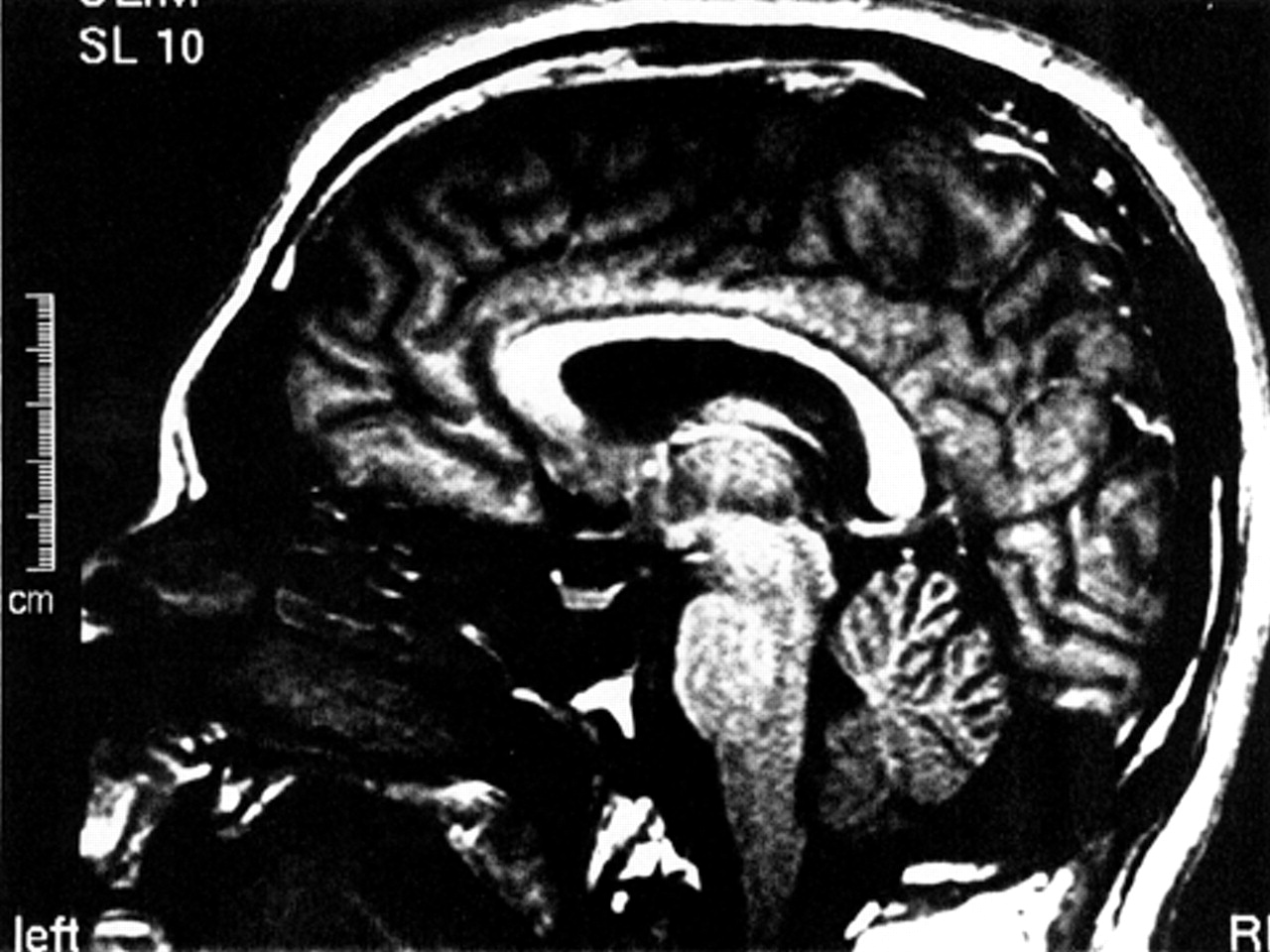
An MRI scan of A.A.’s brain, done 6 weeks into the admission, revealed ill-defined intensities and flair areas affecting both cerebellar hemispheres (
Figure 2). This abnormality extended into the right brachium pontis, but no edema or mass effect was observed. There was an increase in the depth of the folia of the cerebellum, and a mild increase in the size of the third and lateral ventricles. There were tiny subcortical hyperintensities in the right insula and frontal lobes bilaterally. An extra-axial lesion was located in the right posterior fossa, causing minimal mass effect and showing characteristics of an arachnoid cyst. The neuroradiologist suggested the possibility of an undefined neurodegenerative process. A second reading of the scan speculated that the cerebellar lesions may be consistent with ischemic injury.
Cognitive Assessment
An intellectual assessment conducted at age 20 revealed intellectual abilities in the borderline-impaired range. Neuropsychological testing was also performed 6 weeks into last admission, with A.A. on a combination of antipsychotic and mood stabilizer medications. A history of cognitive difficulties since childhood was reported by A.A., including delayed language acquisition. He was in special education classes for most of his academic career, which ended after the eighth grade. He also described several years of speech therapy and reported specific learning difficulties in reading and spelling. Indeed, during the cognitive assessment, speech anomalies were noted which included a mild dysarthria accompanied by mild to moderate word finding difficulties and stuttering. It was also noted that A.A. became “breathless” when faced with challenging tasks.
Neuropsychological assessment included measures of intellectual abilities (WASI), verbal (California Verbal Learning Test) and spatial (Rey-Osterrieth Complex Figure) learning and memory, motor speed (Halstead-Reitan finger tapping), fine prehension (Grooved Pegboard, Reitan and Davidson, 1974),
45 and executive functions (verbal fluency, card sorting, verbal and visual abstraction, working memory).
Results showed significant differences from the mean (Z > two standard deviations from sample mean for age and, when available, education corrected norms) in processing verbal material which affected many functional spheres, including measures of general intellectual ability. Results from the language tests showed significant impairments in naming, reading, word knowledge and impaired verbal abstraction. There was a discrepancy between his verbal memory ability, which was characterized by poor learning, retention and retrieval, and his nonverbal memory, which was in the low-average range. Attention for auditory stimuli was two standard deviations below population norms both for simple focused attention and more complex divided attention. The attention difficulties were also observed with visually based tasks requiring sustained divided attention. Measures of fine motor skills, speed and agility showed clear evidence of impairment. Executive functions were also slightly worse in the verbal than visual domains, but the borderline-impaired results in the visual domain denote difficulty with hypothesis generation and abstraction of even simple relationships between concepts. Although performance was in the average range for word generation, errors included rule breaks (out of category words) and repetitions (poor monitoring). The performance on the sorting task was in the low-average range, but should be interpreted with caution, as A.A. had been previously exposed to this task, which is highly sensitive to practice effects.
Individuals with cerebellar pathology usually show impaired visuospatial abilities, impaired verbal fluency, inappropriate behavior (that is constant, not sporadic), and language deficits such as agrammatism and dysprosody. However, A.A. showed greater relative proficiency with visual than verbal material. Fluency was average, and overall results are incongruent with those described by Schmahmann and Sherman.
18 There were some findings of cerebellar involvement, including sequencing difficulties, slowed motor output, and some findings on tests of executive function. This argues in favor of a cerebellar contribution, but the argument is weak based on the neuropsychological assessment and may be related to the insular and frontal lesions.
DISCUSSION
This case mirrors several interesting areas of schizophrenia research, alongside current pursuits in cognitive neuroscience, neurology and genetics. Research findings of the cerebellar contribution to cognition, cerebellar abnormalities in psychosis and schizophrenia, and the presence of intermittent ataxias, some being associated with psychosis, are manifest clinically, cognitively, and neuroanatomically in A.A.’s case. Since the episodic ataxic states appear to be present to some degree in many, if not all, of A.A.’s episodic psychotic states, a strong argument can be made for the cerebellum as a shared neuroanatomic correlate for both conditions.
With regard to neurodegenerative processes, Knoll et al.
33 reviewed evidence and found the data consistent with an ongoing neurodegenerative disease in a significant subgroup of schizophrenic patients. Progressive increases in ventricular size, cell membrane changes, tissue loss, premature neurophysiological and neurochemical changes, and progressive delays in treatment response were found in this subgroup, suggesting an etiologically distinct form of psychosis. Lieberman
34 also argued that diverse lines of investigation support more than one pathophysiologic process and that one subgroup may be “neurodevelopmental,” having a severe form of illness with more treatment resistance. It remains unclear whether the prodromal, active and/or residual phase of illness or the
duration of untreated psychosis is actually neurodegenerative.
Andreasen et al.
35 argued that schizophrenia could be a single disorder explained entirely by a neurodevelopmental mechanism. They propose that neural misconnections, secondary to abnormal brain development, lead to a fundamental cognitive deficit that impacts all spheres of cognition, emotion, and behavior. They do acknowledge the challenging distinction between development and degeneration. The Andreasen group go on to provide evidence for disruption in a specific neural circuit: the cortical-thalamic-cerebellar-cortical circuit. They suggest that developmental disruption of this circuit leads to a “cognitive dysmetria,” or a fundamental impairment in the synchrony of mental processes.
Perhaps an intermittent physiologic change in the cerebellum, secondary to undefined triggers, which incites the ataxic syndrome, concurrently disrupts the cortical-thalamic-cerebellar-cortical circuit thereby precipitating psychotic symptoms. In fact, A.A.’s MRI findings feature cerebellar, thalamic (third ventricle enlargement) and cortical (insular and frontal hyperintensities) involvement, potentially highlighting the cortical-thalamic-cerebellar-cortical circuit in its entirety. Evidence relating this potential ataxic-psychotic disorder to a calcium channel pathology has been presented, but the cause for the periodicity, or activation of the changes, is not clear.
The observation of a breathing dysrhythmia, noted to be a fluctuant rhythm similar to Cheyne-Stokes respiration by one neurologist, may implicate brain stem abnormalities in A.A.’s collection of findings. Brain stem abnormalities of the isodendritic core,
36 pons,
8 and reticular formation,
37 along with several other nuclei, have been implicated in psychosis and schizophrenia. As well, gliosis in the reticular formation, which contains neurons involved in respiration, may occur secondary to chronic hypoxia.
38 Perhaps related to A.A.’s case is the description by Joubert et al.
39 of four patients with episodic hyperpnea, abnormal eye movements, ataxia, and mental retardation associated with cerebellar vermis abnormalities. These researchers report on 16 other similar case descriptions with combined vermis and/or hemispheric cerebellar abnormalities, six of which reveal similar findings to A.A. Perhaps another clue relevant to A.A.’s history is reported by Yamaguchi et al.
40 They described a 17-year-old mentally retarded man (IQ=70), with CT findings of mega cisterna magna and cerebellar hypoplasia and who developed psychotic symptoms at age 14.
Further, A.A.’s findings overlap with intermittent or periodic forms of ataxia. Perez-Rodriguez et al.
41 described a family who suffers from acute intermittent familial ataxia, with symptoms of gait ataxia, dysarthria, and somnolence of acute onset, often precipitated by emotional or physical stress, lasting hours to 5 or 6 days. Biochemical, physiologic, and imaging studies were negative, and symptoms were relieved with acetazolamide. Neufeld et al.
42 described four cases of acetazolamide-responsive periodic ataxia, with recurrent episodes of vertigo, cerebellar ataxia, and nystagmus. They state that the disorder is now characterized genetically, and reviewed EEG findings in 54 of 140 published cases (18 relatives and nine sporadic cases), finding nonspecific abnormalities in 54%. They concluded that the disorder is probably not epileptic. One other case study of note describes a 39-year-old man with intermittent cerebellar dysfunction (ataxia, nystagmus, dysarthria, and vertigo) since the age of 10 who was given a diagnosis of episodic ataxia. Episodic ataxia has been associated with mutations in the CACNL1A4 gene, which is also affected in familial hemiplegic migraine and spinocerebellar ataxia type 6.
43The results of A.A.’s neuropsychological testing only weakly support a cerebellar contribution. It may be that the testing delineated his baseline function, or his nonpsychotic and nonataxic state, gleaning a general neurodevelopmental problem affecting many brain areas. The cerebellar contribution to cognition may have therefore been drowned out in the midst of multiple and global abnormalities. Perhaps only during an
episode of cerebellar malfunction will the cerebellar contribution to cognition reach the point of superseding his baseline deficits to become prominent and observable. Conceivably only then is enough neural circuitry, or the
essential neural circuit, disrupted, thereby permitting the psychotic break and its cognitive sequelae. Reports of cerebellar pathology in childhood
13 show remarkable recovery of function with development. Indeed, A.A.’s cerebellar dysfunction most likely dates to childhood, and he may have therefore compensated for these deficits over time, although with some manifestations still measurable.
In summary, A.A.’s case is consistent with a neurodevelopmental syndrome, representing a unique neurologic variation of schizophrenia and psychotic illness. Support of the cerebellum-psychosis association in A.A. is strengthened through research findings of CAG expansion mutations in the CACNL1A4 gene, which may genetically conjoin intermittent cerebellar dysfunction, neurocerebellar syndromes, and psychotic illness. Etiologically, the case argues for a novel concept of neurodevelopmental heterogeneity. It is possible that disparate mutations in the calcium channel gene are responsible, thereby causing an uneven disruption of various motor, cognitive, emotional and perceptual symptomology. The cortical-thalamic-cerebellar-cortical circuit may be involved, with the cerebellar component being the primary disturbance in A.A. As well, the breathing dysrhythmia may support a possible brain stem contribution. Triggers for the periodicity of his neurologic and psychotic symptoms are likely multifactorial and remain poorly understood at this time.