The usefulness of the routine electroencephalogram (EEG) in psychiatric practice and research is significantly hampered by the reported prevalence of EEG abnormalities in “normal” adult populations, ranging from 4% to as high as 57.5%.
1 This wide range likely reflects the lack of clear and rigorous standards for choosing subjects for healthy comparison groups. Blanc et al.
2 documented that the inclusion of psychiatric patients in healthy comparison samples contributes to increased prevalence of EEG abnormalities in the examined sample. This observation was reported as early as 1939
3 and remains unchallenged today. In order for this technique to be reliable, the boundaries of normality should be well defined.
The unquantified EEG remains the sole technique that can confidently detect cerebral epileptiform discharges.
4 Automated spike detection, while rapidly progressing, remains dependent on the visual inspection for the final verification of the abnormal nature of events detected by the computer.
5,6 Automated spike detection has more accuracy in detecting seizures than single interictal epileptiform discharges.
7 Moreover, the controversial waveforms (see below) are characterized by being small in amplitude and require specialized training for a clinician to confidently recognize them. The unquantified EEG is also capable of detecting significant focal slowing indicative of structural abnormalities and generalized slowing indicative of diffuse pathology. Spectral EEG analysis (quantified EEG) is a complementary technique that can detect EEG deviations beyond what visual inspection can detect.
8 For the last two decades, EEG research in psychiatry has relied heavily on the quantified EEG with almost a complete lack of unquantified EEG research. Unquantified EEGs (whether in an analog or digital format) are readily available in all clinical and most research institutions. The unquantified EEG (we will use the term “routine EEG” to refer to unquantified EEGs in the remainder of this paper) can be used to evaluate a number of neuropsychiatric presentations for clinical purposes and can be used to exclude research subjects with EEG abnormalities in order to increase the homogeneity of study samples.
The nature of comparison subjects in psychiatric research is a crucial element that can significantly influence the results of biological investigations.
9 With the advent of the Research Diagnostic Criteria and, more recently, the Structured Clinical Interview for DSM (SCID),
10 it is now possible to select healthy comparison subjects in a more standardized fashion without much overlap with groups of patients with psychopathology. The danger of reliance on a subject’s self-report of normalcy was highlighted by Halbreich et al.,
9 who showed that in a sample of self-proclaimed “normal volunteers,” 16.5% met criteria for diagnosis of a current mental disorder and of the subjects without a current mental disorder, as many as 35% had past histories and 39% had family histories of mental illness. This is particularly important because physiological differences have been found between “normal subjects” with and without family histories of mental disorders.
11,12Inclusion and exclusion criteria used for patient selection have also been progressively more restrictive, particularly for imaging and physiological studies. The presence of general medical or neurological conditions that may influence particular measurements are routinely used as exclusion criteria. Whether a subject is receiving medications that may affect brain functions is either exclusionary or well controlled for in the majority of studies published in psychiatric peer-reviewed literature during the last decade or longer and is an essential requirement for most granting agencies. Furthermore, issues of drug abuse and dependence, presence of axis II disorders, and family history of psychiatric disorders are frequently controlled for based on the specific study question. Control for such variables is unlikely to result in a “super normal” comparison group as many of these criteria are frequently required in patient groups as well.
In no place is the establishment of normal EEG boundaries as important as in the wave forms (i.e., 14 and 6 positive spikes, small sharp spikes, psychomotor variant, and 6/second spike and waves) of which the clinical significance remains controversial.
13In this review, the existing literature defining the boundaries of the normal unquantified EEG was examined in order to assess whether or not our current knowledge can be considered adequate for neuropsychiatric research purposes.
METHODS
An extensive search of the literature included in Medline and PsychInfo databases for all articles listing EEG as a keyword was performed. Textbook chapters discussing “normal EEG” were also examined for references.
14 These two sources were the primary sources for references. The reference lists of each paper or book chapter were searched for older relevant papers. Articles pertaining to quantified or spectrally analyzed EEG, articles examining evoked responses or sleep EEG, and articles that did not include normal subjects or did not study humans were excluded from the study. Additionally, review articles and abstracts were excluded.
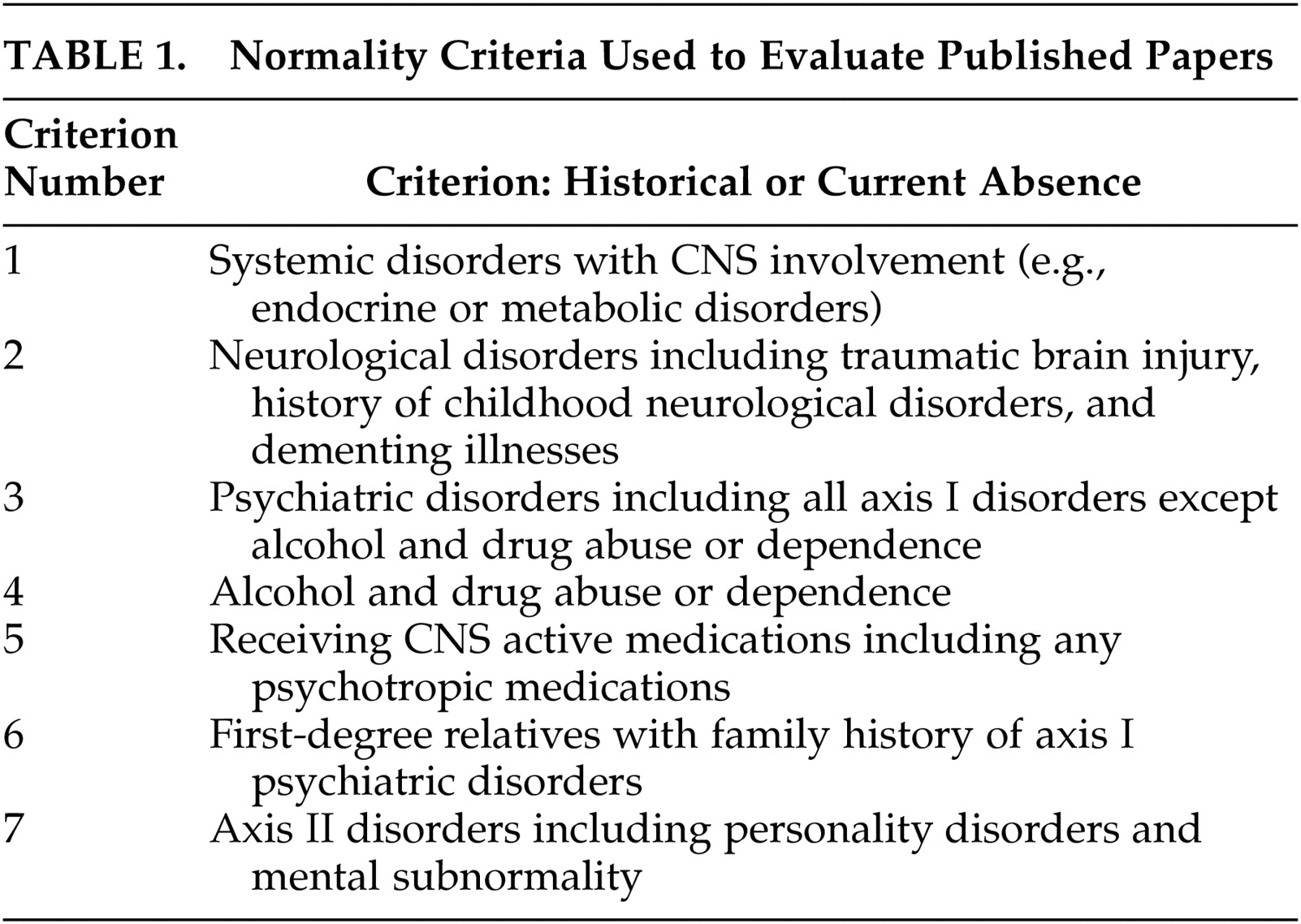
Two authors (N.B. and H.M.) independently performed a thorough examination of the Methods section of each article, searching for the criteria of normality taken into consideration. Seven criteria that are commonly used in contemporary neuropsychiatric research for selecting healthy comparison subjects were chosen as the bases for this review (
Table 1).
Criteria were ranked as present, absent or unclear for each article. When one of the two reviewers marked a criterion as “unclear,” the two reviewers jointly reviewed the methods section to render a final determination. We then determined that an “unclear” category was not of value for the purposes of this review. Thus, if a criterion could not be confidently rated as present by both evaluators, the criterion was marked as absent. As the majority of studies identified by this search were performed prior to the publication of the DSM criteria, the simple mention of a criterion was credited as present. A notably large number of studies simply stated that subjects were “healthy.” These studies are starred in
Table 2. The simple assurance of normality was considered inadequate for our purpose, and they were marked as lacking all 7 normality criteria. While this is unlikely to have been the case, we elected to err on the side of rigorous reporting. Among the seven criteria were: presence of a general medical condition that may affect the EEG, any neurological conditions including history of head injury leading to any length of loss of consciousness, and history of any psychiatric disorders. History of drug dependence or abuse (excluding tobacco or social use of alcohol), receiving any CNS active medications, family history of psychiatric disorders, and presence of an axis II disorder were also used as criteria for normality. The inclusion of family history as an exclusionary criterion is based on data showing increased EEG abnormality in family members of psychiatric patients.
15The articles were published between 1936 and the present. A total of 38 articles examining the EEGs of “normal” individuals were included. Articles either examined EEGs in “normal subjects” as the sole purpose of the study or included a “normal” comparison group as a comparison for a pathological group. The findings are delineated in
Table 2.
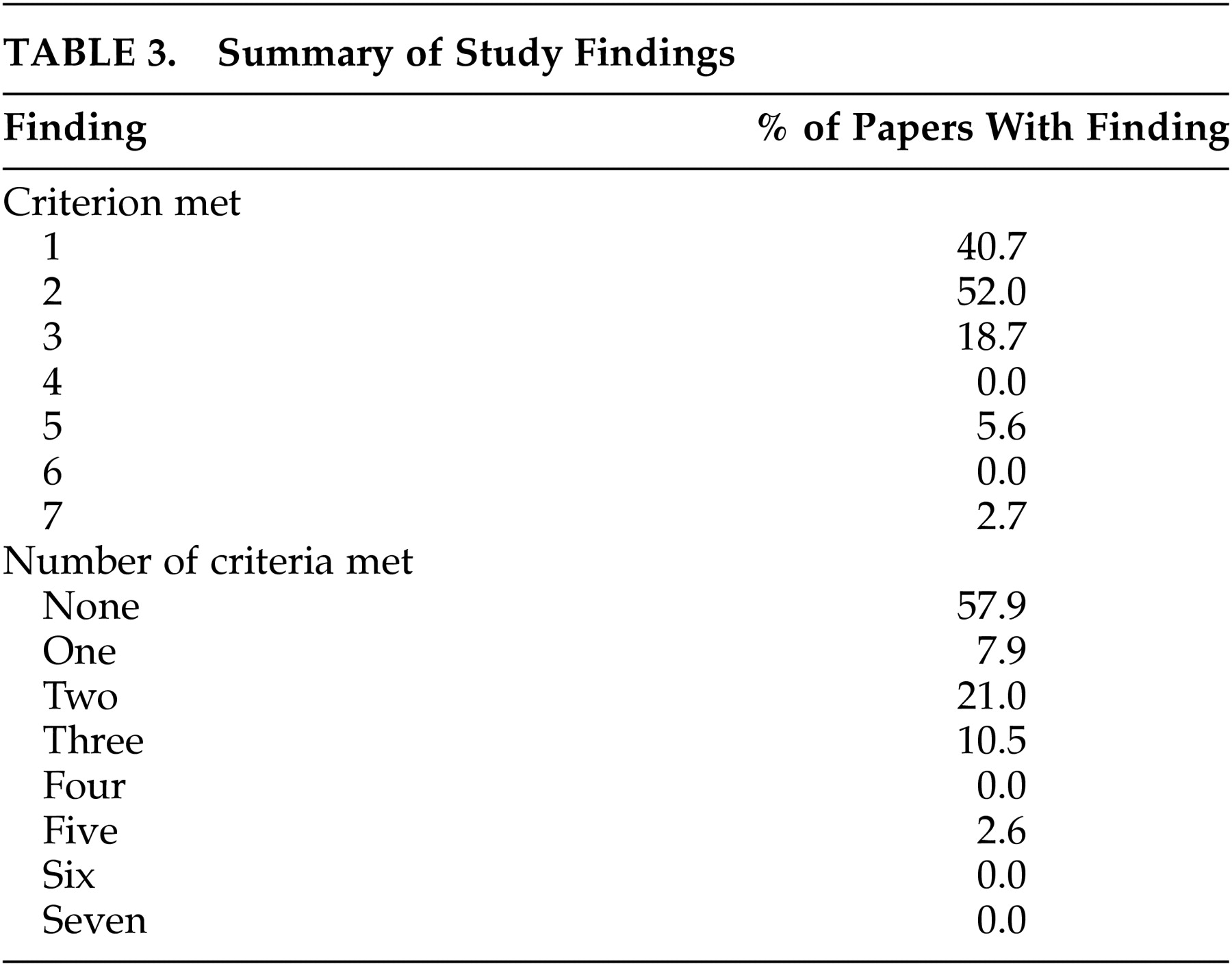
A history or presence of a general medical condition, a neurological psychiatric disorder, or a major psychiatric disorder were three conditions considered to be most important in determining the exclusion of normal subjects. All three criteria were considered to have been met in a total of four studies. Five additional papers limited exclusion criteria to medical and neurological disorders. Excluding subjects solely based on history or presence of a systemic disease that is likely to affect CNS functions (e.g., hypo- or hyperthyroidism) was indicated in two more studies. Exclusion based solely on the presence or history of a neurological problem was seen in one study.
16 In this study, the criterion for excluding subjects based on receiving CNS active medications was also met. Another study excluded subjects based on psychiatric history but without specific criteria for neurological or medical conditions.
15 In this study, a family history of a psychiatric problem was also an exclusionary criterion.
A single study screened for drug abuse.
17 In two studies, subjects receiving psychotropic medications were excluded.
16,18 None of the studies excluded subjects receiving such nonpsychotropic medications as steroids or centrally acting antihypertensive medications. Two papers reported collecting family history data and one other paper reported screening for personality disorders, but these were not considered exclusionary criteria. This is important, as the EEG literature is replete with reports of abnormalities in association with different personality disorders, particularly antisocial and borderline types. Standardized psychiatric scales (e.g., SCID) were not administered in any of the studies. Studies relied mainly on historical denial of psychiatric symptomatology.
Table 3 provides a summary of the above findings.
DISCUSSION AND CONCLUSIONS
The literature review described above indicates that the overwhelming majority of EEG normative studies were performed prior to the advent of normality criteria currently applied by most research institutions in research endeavors involving psychiatric or neuropsychiatric populations. In general, our search indicates that the criteria for normality taken into consideration in currently available literature for “normal” analog EEG range from poor to absent. A neurological history and systemic pathologies impinging on the central nervous system were only implicitly excluded in the majority of studies. The specific exclusion of significant head injury was also lacking. Traumatic brain injury has an annual incidence of 370 per 100,000.
19 Traumatic brain injury can lead to personality changes,
20 affective disorders,
21 or even psychotic syndromes.
22 Medications or psychoactive substances contaminating the picture were not considered as a factor.
The largest studies establishing normality of EEG were conducted on Navy candidates. These studies specifically addressed personality disorders. While subjects were not excluded, data on personality disorders were collected. The importance of rigorous exclusionary criteria was demonstrated by Buchthal and Lennox.
17 In their sample, 5.4% of candidates who were refused admission to the Navy on psychiatric grounds had paroxysmal EEG abnormalities, while only 2.2% who were admitted and completed the training had paroxysmal abnormalities.
One of the major activities of electroencephalographers has been the determination of the incidence of EEG abnormality associated with nervous and mental diseases and the relationships between particular EEG patterns and diseases or their symptoms. Marked relationships have been observed between EEG abnormalities and many neurological disorders, e.g., epilepsy. In terms of neuropsychiatric disorders, the agreement is extremely limited. It does not go beyond the fact that many studies indicate that there is a greater incidence of EEG abnormalities in neuropsychiatric patients. Few generalizations can be made despite the relatively large number of papers dealing with normal routine EEG in psychiatry.
A number of additional factors that contribute to the diminished value of currently existing norms also emerged from our search (specifically, small sample sizes in a number of studies, lack of established criteria for EEG normality, and disagreement among encephalographers regarding the significance of the so-called controversial waveforms).
Based on the above findings, we concluded that the boundaries for normal analog EEG are not well defined for the purposes of neuropsychiatric clinical or research endeavors. In order to be able to better define and study analog EEG abnormalities in neuropsychiatric populations, well designed normative studies are needed.
Specifically, future studies should not rely on a single normal routine EEG to conclude lack of abnormalities. Similarly, the value of securing sleep tracings cannot be overemphasized. A major role of EEG is to reduce the heterogeneity of research studies (e.g., depression with or without localized abnormalities, aggression with or without spikes). In Table 1 we have delineated what we consider adequate factors to be taken into consideration for the inclusion of a subject as normal in a study. Obviously, different types of studies will require different exclusion criteria. For example, studies attempting to develop normative databases or criteria for general use should observe the most stringent criteria. If a person or a group then deviates from such norms, the cause or causes of the deviations can then be investigated in subsequent studies specifically designed to isolate specific possible contributing factors. On the other hand, studies comparing specific patient populations to normal comparison groups may wish to allow some of the factors (e.g., history of drug abuse or head injury) based on study design or be more stringent (e.g., studies examining genetics of an EEG pattern may extend the family history exclusion beyond first-degree relatives).
Blanc et al.
2 highlighted the problem of cross-sectional studies. Via case examples, they pointed out that EEGs may change its characteristics at different time points. They related these changes mainly to change in psychiatric status. Additionally, Chamberlain and Russell
15 pointed out that first-degree relatives (particularly siblings) of schizophrenia patients may have higher prevalence of EEG abnormalities. This finding suggests that family history of axis I disorders (at least in first-degree relatives) should be an additional exclusion criterion for normative studies.
The above review indicates that the high prevalence of abnormal EEGs in normal populations, ranging from 5% to 20%, is based on inadequate inclusion and exclusion criteria for healthy comparison subjects. We conclude that the boundaries for EEG normality are poorly defined as they currently stand and are invalid for drawing any conclusions regarding prevalence or significance of EEG abnormalities in psychiatric populations. The EEGs of large samples of well characterized healthy individuals meeting the criteria specified in
Table 1 need to be examined in order to provide more clearly defined boundaries of normality and to establish more uniform criteria for abnormality.
While all efforts were made to obtain all published papers and book chapters addressing the development of normative EEG criteria, it is unavoidable that a number of such publications were not securable (most of this literature dates back 40–60 years). Similarly, it was not possible to contact the individual investigators to verify normality criteria used (for the same reason). Nonetheless, the data presented above strongly suggest the need for new research to help define the normal boundaries of the unquantified EEG.
ACKNOWLEDGMENTS
This study was supported by the VA-Connecticut Healthcare System and presented at the 9th Annual Meeting of the American Neuropsychiatric Association, 1998, Orlando, Florida.