The wake-promoting agent modafinil is an effective and well-tolerated treatment for the improvement of wakefulness in patients experiencing excessive sleepiness associated with narcolepsy.
1,2,3 Although the precise mechanism of action of modafinil has not been established, findings from preclinical studies suggest that modafinil may promote wakefulness through its selective action on areas of the brain responsible for maintaining normal wakefulness.
4,5,6In two 9-week, large-scale, double-blind, placebo-controlled clinical trials, once-daily administration of modafinil at doses of 200 mg and 400 mg has been shown to significantly enhance wakefulness through mid-afternoon.
1,2 The relatively long elimination half-life of modafinil (12 to 14 hour)
7 further supports a once-daily dosing regimen.
Despite these clinical and pharmacokinetic observations, some patients with narcolepsy who have satisfactory responses to modafinil in the early part of the day may experience sleepiness in the late afternoon or evening. Significant improvement in wakefulness has been demonstrated with split-dose regimens of modafinil in three previously conducted studies,
3,8,9 but comparative efficacy versus once-daily regimens was not established. We recently completed two similar randomized, double-blind, parallel studies comparing the relative effects of various modafinil doses and dose regimens on wakefulness throughout the entire waking day in patients with narcolepsy experiencing residual late-afternoon/evening sleepiness following satisfactory responses earlier in the day. Here, we present a combined analysis of data from these two studies.
RESULTS
Patients
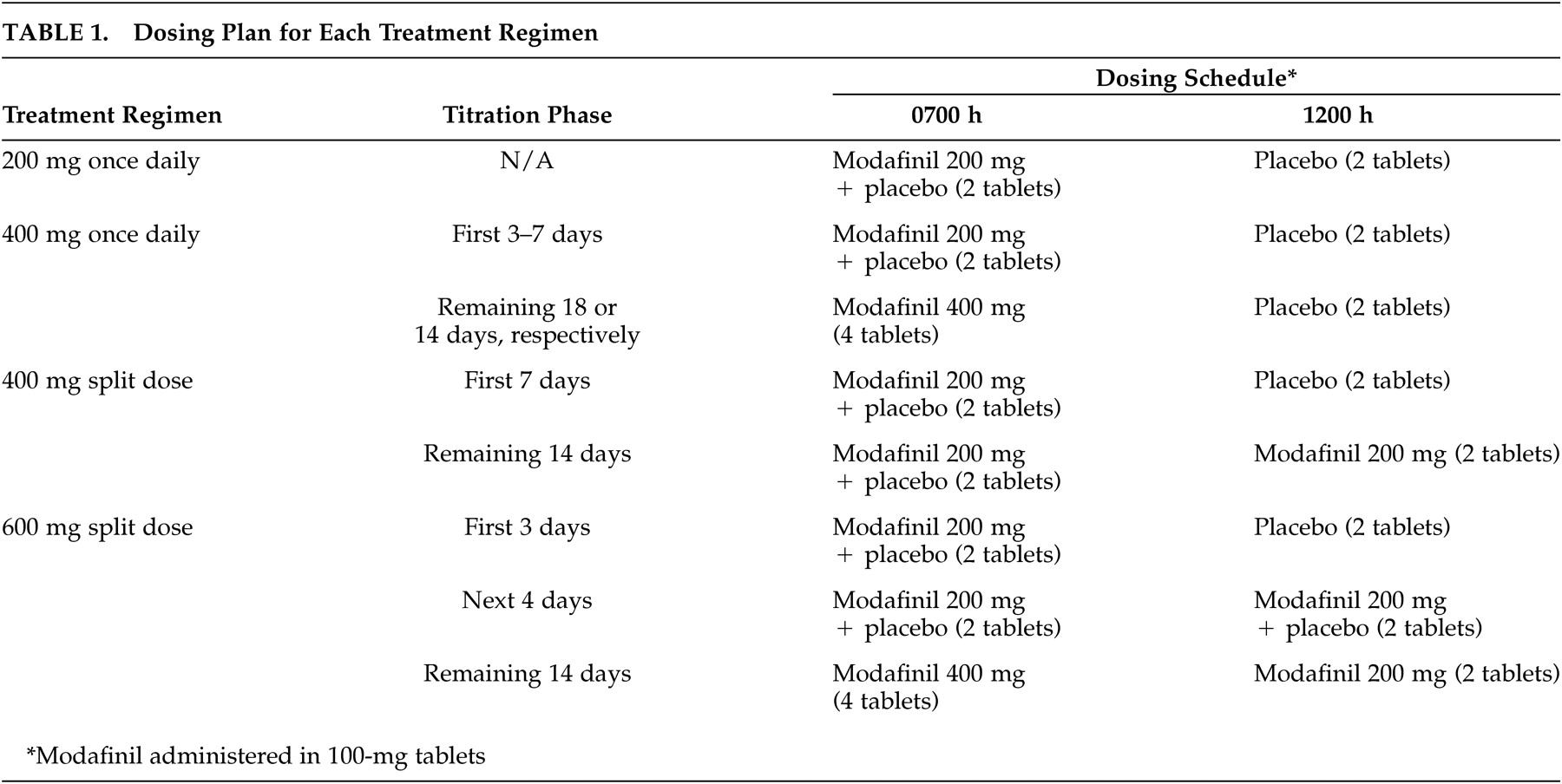
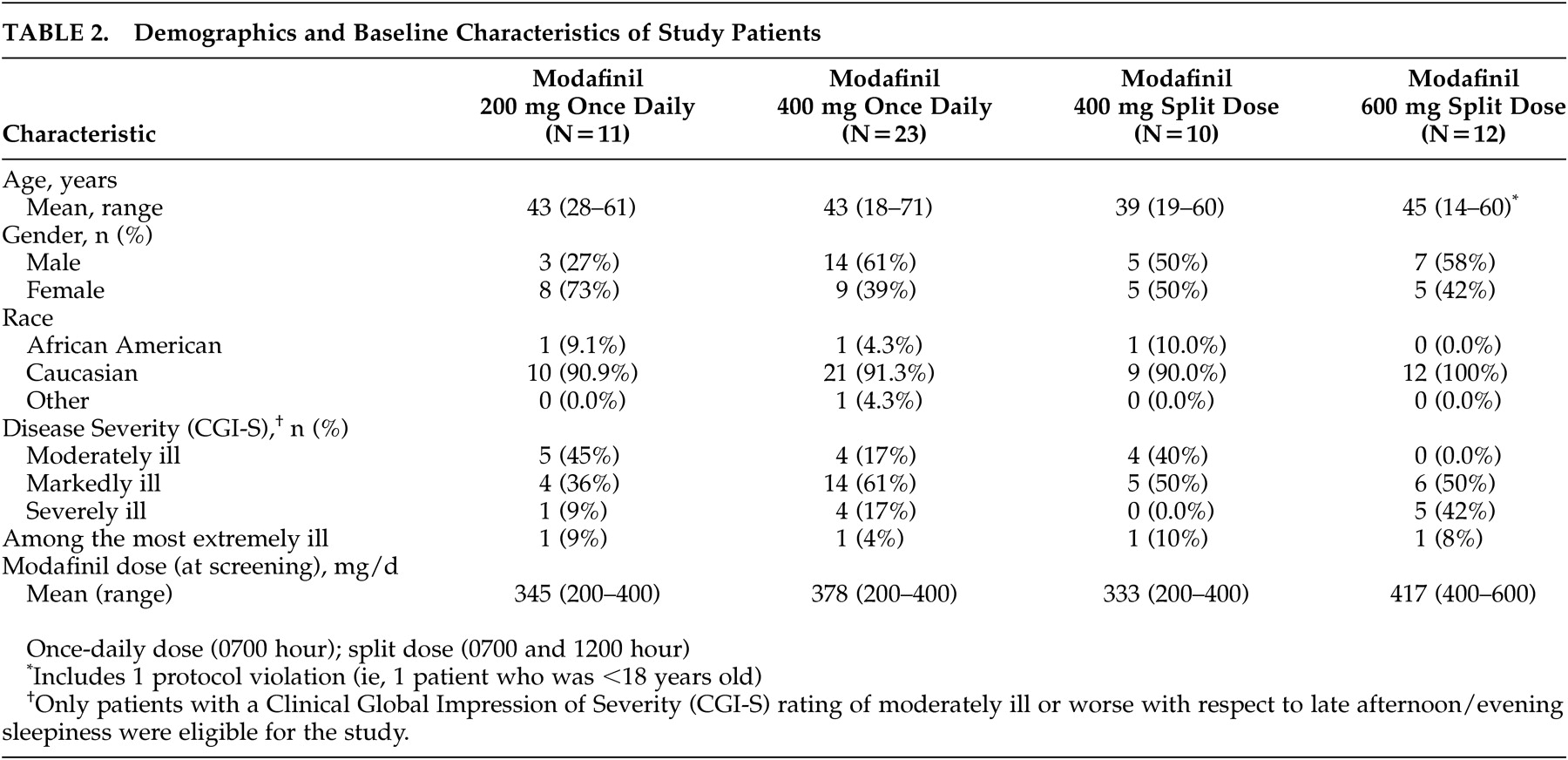
Of the 56 patients randomized to treatment, 11 received modafinil 200 mg once daily, 23 received modafinil 400 mg once daily, 10 received the modafinil 400-mg split-dose regimen, and 12 received the modafinil 600-mg split-dose regimen. The demographic and baseline characteristics of the patients in the four treatment groups were comparable (
Table 2). Patients’ ages ranged from 14 to 71 years (mean ∼43 years). The majority (N=52; 93%) of the study patients were white, and slightly more than one-half were males (N=29; 52%). Most patients (77%; N=43) were markedly, severely, or extremely ill. All 56 patients completed the 3-week double-blind study period.
Efficacy Assessments
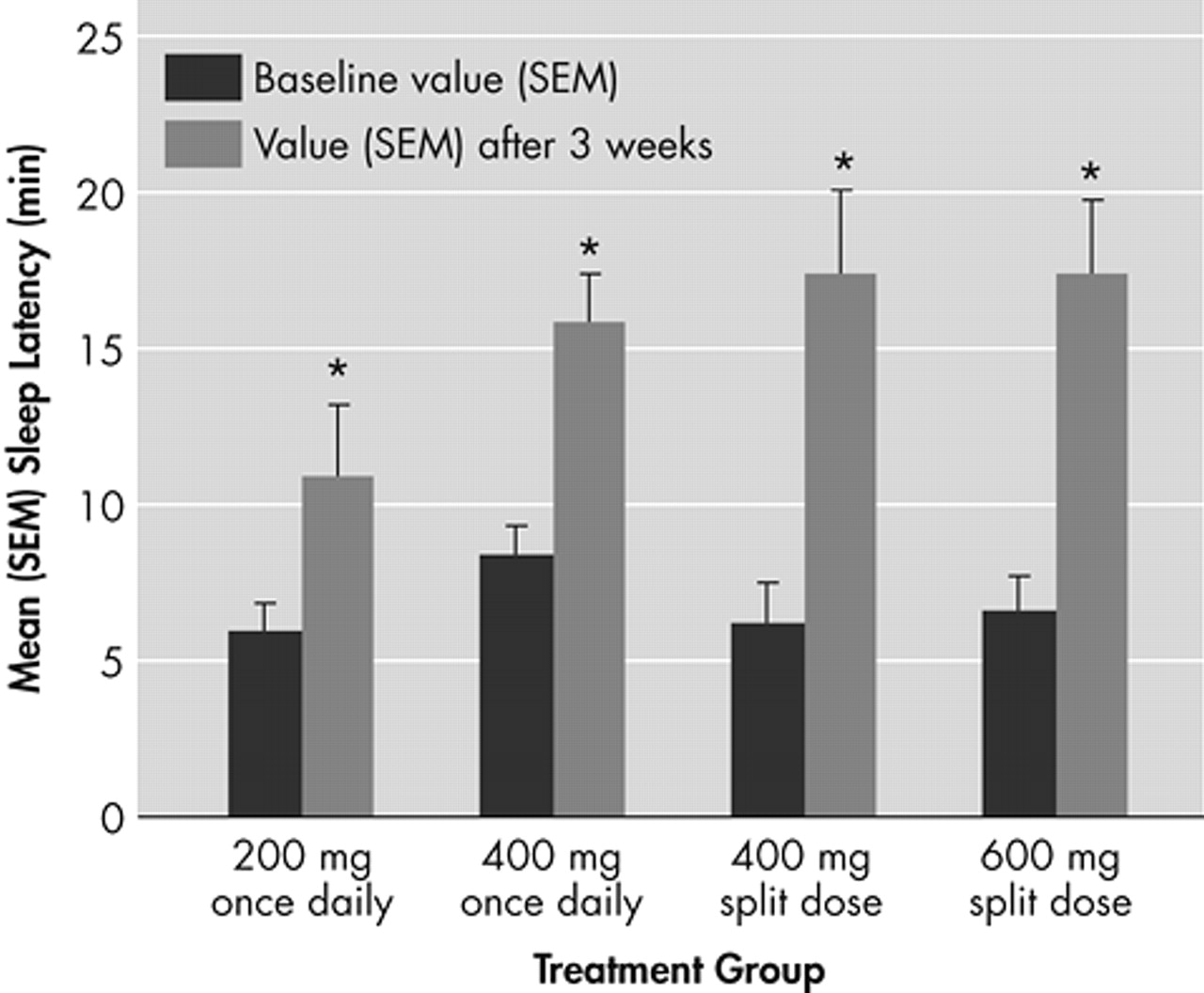
All modafinil doses and dosing regimens significantly improved patients’ ability to sustain wakefulness based on the total daily mean MWT sleep latency times at week 3 compared with baseline (p<0.01) (
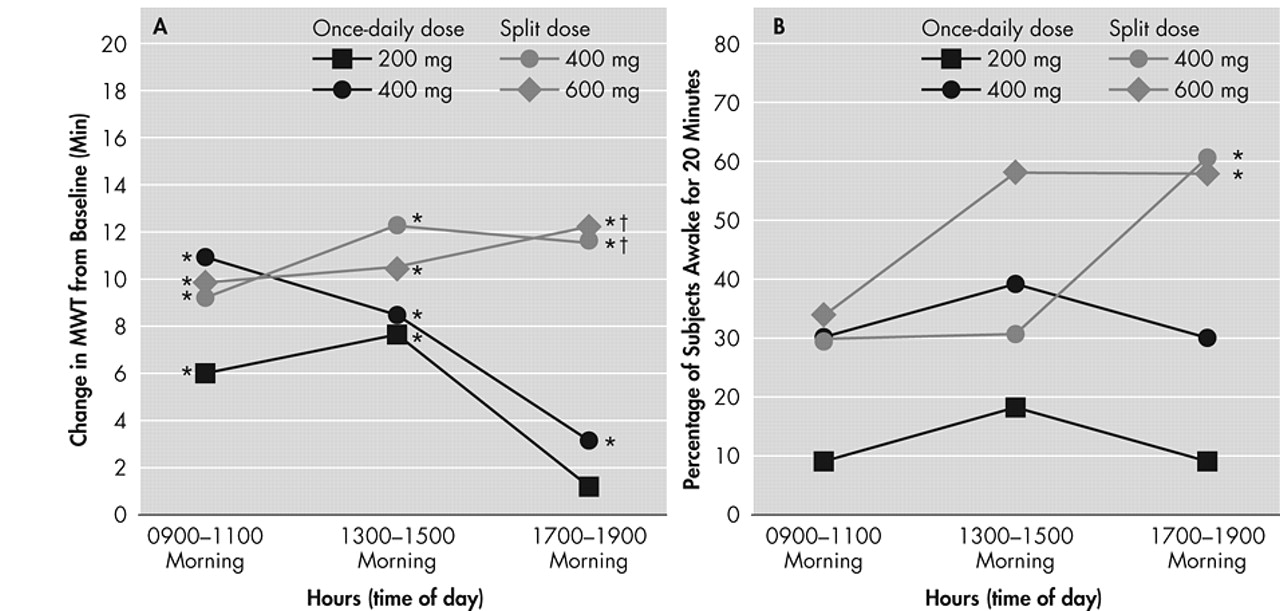
Figure 1). Dose response effects were seen (p<0.05). With respect to late afternoon/evening sleepiness (1700 to 1900 hour), each of the two split-dose regimens of modafinil produced significantly greater mean improvements from baseline in MWT sleep latency (p<0.05) than the 200-mg once-daily regimen (
Figure 2, Panel A).
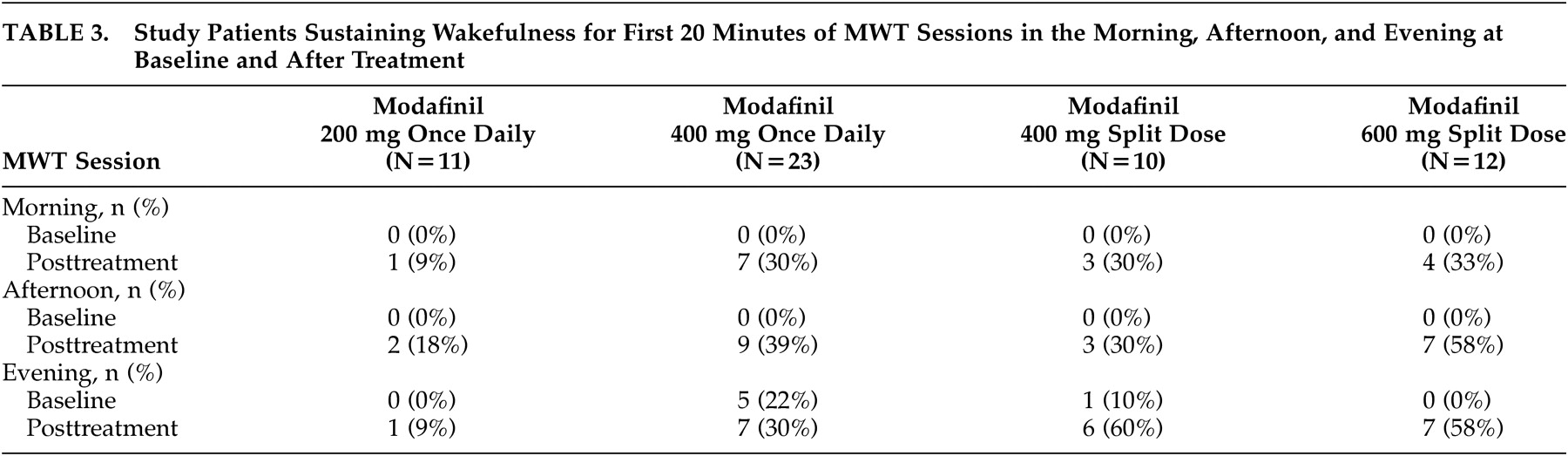
At baseline, only 11% (6 of 56) of all patients were able to remain awake for the first 20 minutes of the MWT sessions in the evening (
Table 3). After 3 weeks of modafinil treatment, the percentage of patients across the 4 treatment groups who were able to sustain wakefulness for the first 20 minutes of the MWT evening sessions was significantly increased to 38% (21 of 56, p<0.05). A significantly higher proportion of patients receiving the modafinil 600-mg split-dose or the 400-mg split-dose regimens were able to sustain wakefulness for at least 20 minutes during both MWT sessions in the evening, compared with those receiving the 200-mg once-daily dose regimen (p<0.05) (
Figure 2, Panel B).
At placebo-baseline, none of the study patients were able to sustain wakefulness for the first 20 minutes of the MWT sessions in the morning or afternoon. After 3 weeks of treatment, the modafinil 600-mg split-dose group had the highest proportion of patients able to sustain wakefulness for the first 20 minutes of the MWT sessions in the morning and afternoon, followed by the modafinil 400-mg split-dose, 400-mg once-daily, and 200-mg once-daily groups (
Table 3).
After 3 weeks of treatment, all modafinil dosing regimens improved wakefulness, as demonstrated by reductions in mean ESS total scores from baseline. Significant improvement from baseline in mean ESS scores was observed in patients receiving the modafinil 200-mg (p<0.05) and 400-mg (p<0.0001) once-daily and 600-mg (p<0.05) split-dose regimens.
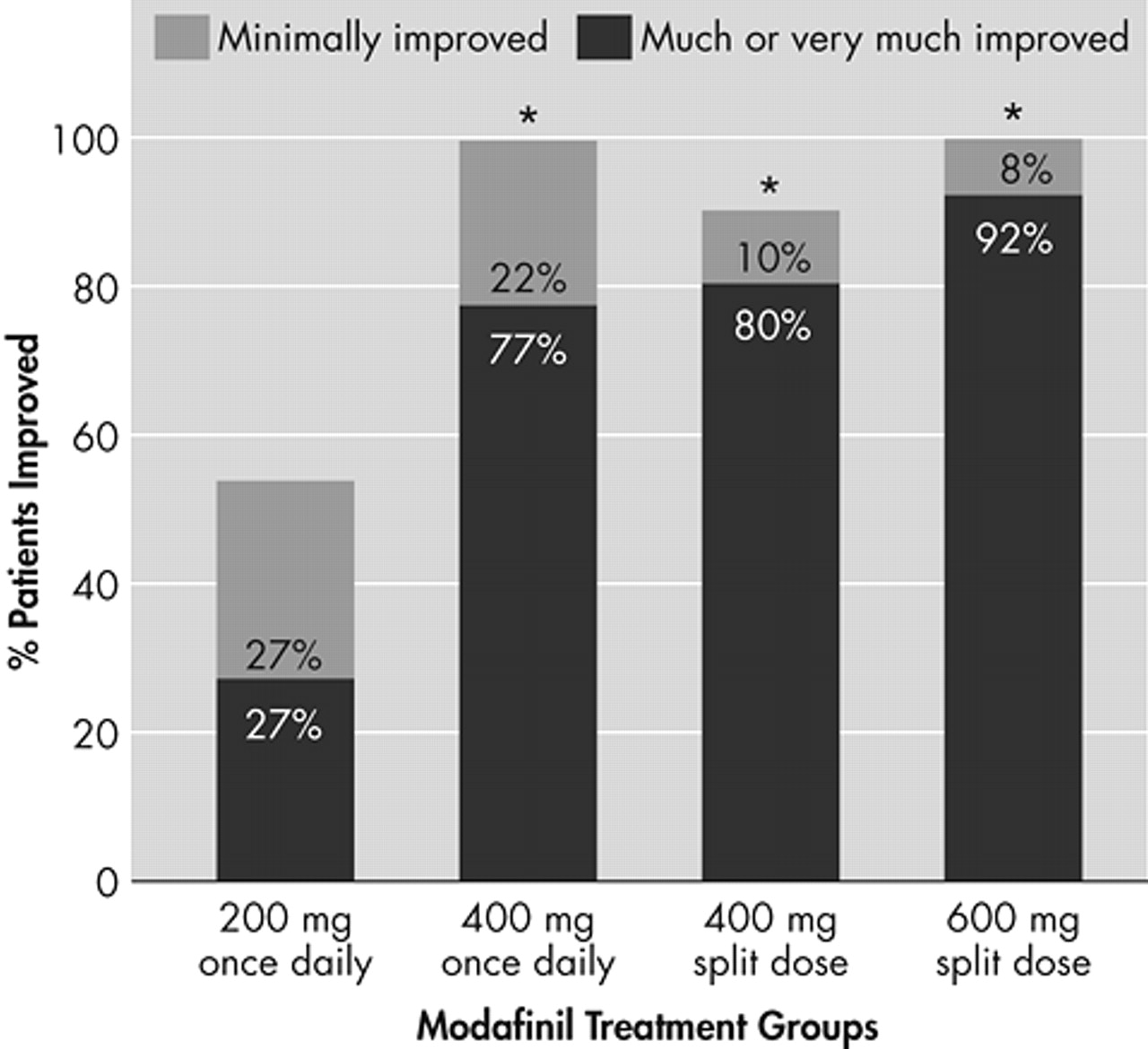
All four modafinil dosing regimens improved the overall clinical condition (CGI-C) after 3 weeks of treatment, relative to baseline (
Figure 3). With respect to late-afternoon/evening sleepiness, the proportion of patients rated as “much improved” or “very much improved” was highest among those receiving the modafinil 600-mg and 400-mg split-dose regimens: 92% and 80%, respectively, compared with 70% of patients in the 400-mg once-daily group and 27% of patients in the 200-mg once-daily group. The proportion of patients rated as being at least “improved” was significantly higher in the modafinil 600-mg split-dose (100%), 400-mg split-dose (90%) and the 400-mg once-daily (91%) groups than in the 200-mg once-daily group (55%, p<0.01).
Safety Assessments
All modafinil dosing regimens were well tolerated. A total of 10 patients reported adverse events: seven receiving modafinil 400 mg once daily and one each receiving modafinil 200 mg once daily, modafinil 400 mg split dose, and 600 mg split dose. All adverse events were mild or moderate in severity. No serious adverse events were reported, and no patients discontinued the study because of adverse events. One patient receiving 400 mg once daily did experience adverse events (mild-to-moderate agitation, irritability, nervousness, anxiety, gastrointestinal distress, and insomnia) that led to temporary discontinuation of modafinil; however, the patient continued with the established protocol. The most common adverse events assessed by the investigator(s) as being possibly or probably related to treatment were gastrointestinal events (nausea and dyspepsia), which occurred in a total of three patients (5%) who received once-daily regimens of modafinil 200 mg or 400 mg. Other adverse events potentially related to modafinil were headache (N=1) and emotional lability (N=1), which occurred with the 400-mg once-daily regimen, and anxiety (N=1), which occurred with the 200-mg once-daily regimen (overall incidence of 2% for each adverse event). Results of laboratory tests, physical examinations, vital sign measurements, and ECG recordings indicated no clinically significant treatment-related abnormalities.
DISCUSSION
The investigations included in this analysis were the first to directly compare the efficacy of once-daily and split-dose regimens of modafinil in improving wakefulness throughout the entire waking day in patients with narcolepsy who experienced a positive response to modafinil early in the day but had subsequent late-day sleepiness. The findings of the MWT showed that 600 mg of modafinil administered as a split dose and 400 mg administered either once daily or as a split dose were significantly more effective in maintaining wakefulness throughout the day than 200 mg once daily in this population. Significant improvements in clinical condition (CGI) with respect to evening sleepiness further demonstrated that the higher once-daily dose and split-dose regimens were significantly better than the 200-mg once-daily dose in facilitating wakefulness throughout the day. All four dosage regimens of modafinil (200 mg once-daily, 400 mg once-daily, 400 mg split-dose, and 600 mg split-dose) significantly improved daytime wakefulness and overall clinical condition compared with baseline. Modafinil was generally well tolerated, with no apparent differences in the frequency or severity of adverse events among dosing regimens and no treatment discontinuations because of adverse events.
Although once-daily and split-dose modafinil regimens have not been directly compared in previous clinical trials, the efficacy of different modafinil doses and dosing regimens has been previously evaluated. These findings suggest that 400 mg once daily is superior to 200 mg once daily in maintaining wakefulness throughout the day. In contrast, in two large-scale clinical trials, no statistical difference in efficacy was found between 200-mg and 400-mg modafinil once-daily doses.
1,2 However, the latter studies included a different study population, were not designed to assess late-day wakefulness, and employed distinct testing procedures. The previous trials included modafinil-naive patients,
1,2 whereas the present study included patients who were stabilized on higher mean doses of modafinil (approximately 368 mg/day). In these previous trials, modafinil was administered approximately 1 hour prior to the first MWT session, and MWT assessments were discontinued in the early afternoon. As modafinil serum levels peak 2 to 4 hours after administration, the testing schedule used in the present study protocol offered a greater opportunity to discriminate between the effects of the 200-mg and 400-mg doses over a longer time period. By using an extended MWT protocol, we were able to capture differences in response between the tested doses and dose regimens in the evening (1700–1900 hour). Finally, the studies included in this analysis employed 30-minute rather than 20-minute MWT sessions, thus minimizing the potential for a ceiling effect.
16In the present study, we found significant improvement in late-afternoon and evening sleepiness with both the 400-mg and 600-mg split-dose regimens of modafinil. Broughton et al. demonstrated dose-related differences in efficacy between a 200-mg split-dose and a 400-mg split-dose regimen.
3 In their study, which used a 40-minute MWT session, the 400-mg split dose significantly improved MWT sleep latency at all testing times (i.e., 0930, 1130, 1330, and 1530 hour), whereas the 200-mg split dose significantly improved sleep latency only at the two testing sessions following the midday dose (i.e., 1330 and 1530 hour). Split-dose regimens of modafinil have also been evaluated in two crossover trials,
8,9 but dose-response differences were not assessed. Patients receiving split-dose modafinil dosing regimens in these trials
8,9 (300 mg/day and 300 mg–500 mg, respectively) demonstrated significant improvement in wakefulness, as assessed by MWT, the ESS, and patient diaries, without adverse effects on nighttime sleep. Similar to our findings, Billiard et al.
9 reported that modafinil 300 mg as a split dose reduced excessive daytime sleepiness, measured by sleep latency on MWT, in the morning (1000 hour) through the early evening (1800 hour).
The results of the present study may have important implications for optimal dosing of modafinil, but several limitations should be considered. Although significant improvements from baseline were demonstrated in objective measures of wakefulness and overall clinical condition for all dosage regimens of modafinil, the small sample size for each treatment group (N≤23) may have limited the detection of treatment differences. Thus, these findings need to be confirmed in controlled trials using larger sample sizes. In addition, the studies included only narcolepsy patients with documented clinical response to modafinil. Because a complex set of genetic, immunological, and environmental factors may influence a patient’s responsiveness to modafinil,
17 the magnitude of the clinical response observed in our study may be exaggerated vis-à-vis the narcolepsy patient population at large. Moreover, as the study included only patients experiencing late-afternoon/evening sleepiness despite satisfactory earlier daytime responses to modafinil, the observed time-of-day effects may not be reproducible in all narcolepsy patients. Nonetheless, the demonstrated advantages of various dose and dose regimens of modafinil for prolonging wakefulness throughout the day may apply to patients exhibiting a treatment response pattern similar to that of the patients in the study sample. Finally, the present studies did not include nocturnal polysomnography or subjective estimates of sleep and sleep quality to evaluate the potential effects of the modafinil dosing regimens on sleep. Therefore, we cannot verify from these studies that the dosing regimens did not interfere with nocturnal sleep. Other studies, however, have reported that 200-mg and 400-mg once-daily doses
1,2 and 200-mg and 400-mg split doses
3 of modafinil did not effect objectively recorded sleep.
Given the emergence of late-day sleepiness in some narcolepsy patients treated with the approved once-daily dose of modafinil, it is apparent that the pharmacokinetics of this agent do not always correlate with its pharmacodynamic effects. In such patients, a twice-daily dose regimen may be more appropriate than the traditional once-daily dose regimen. This pooled analysis revealed that addition of a 200-mg midday dose of modafinil to either 200-mg or 400-mg morning doses is well tolerated and effectively sustained wakefulness throughout the entire waking day in narcolepsy patients who experienced late-afternoon or evening sleepiness with once-daily dose regimens of modafinil.