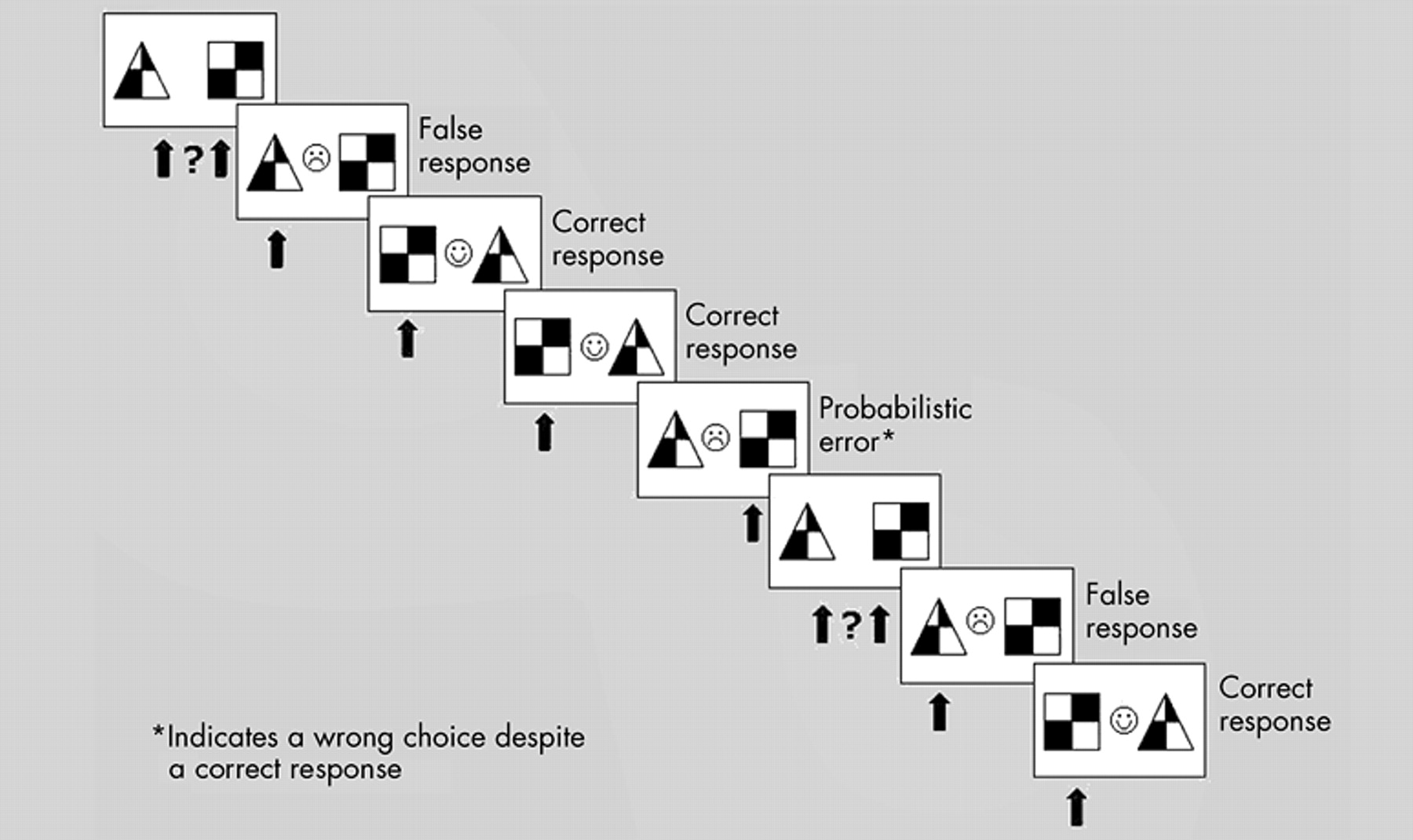
This study compared patients with OCD and healthy comparison subjects on a reversal learning task supposed to activate areas of the fronto-striatal loop, a possible cerebral correlate of OCD.
12,
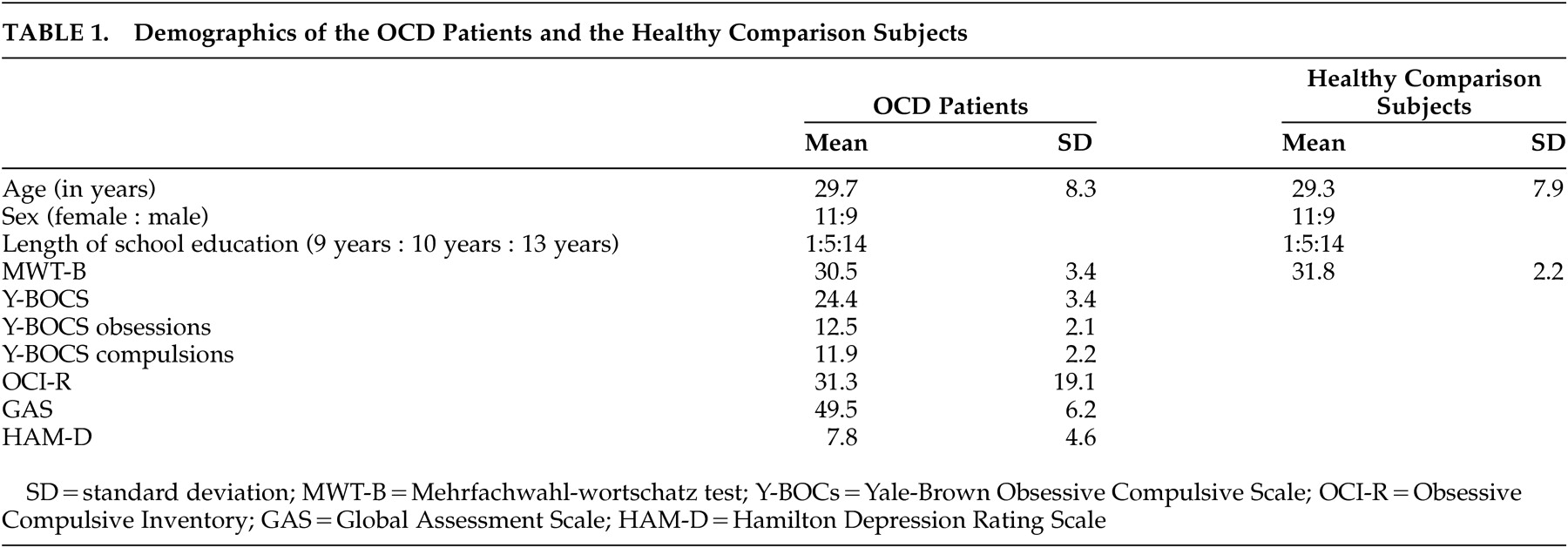
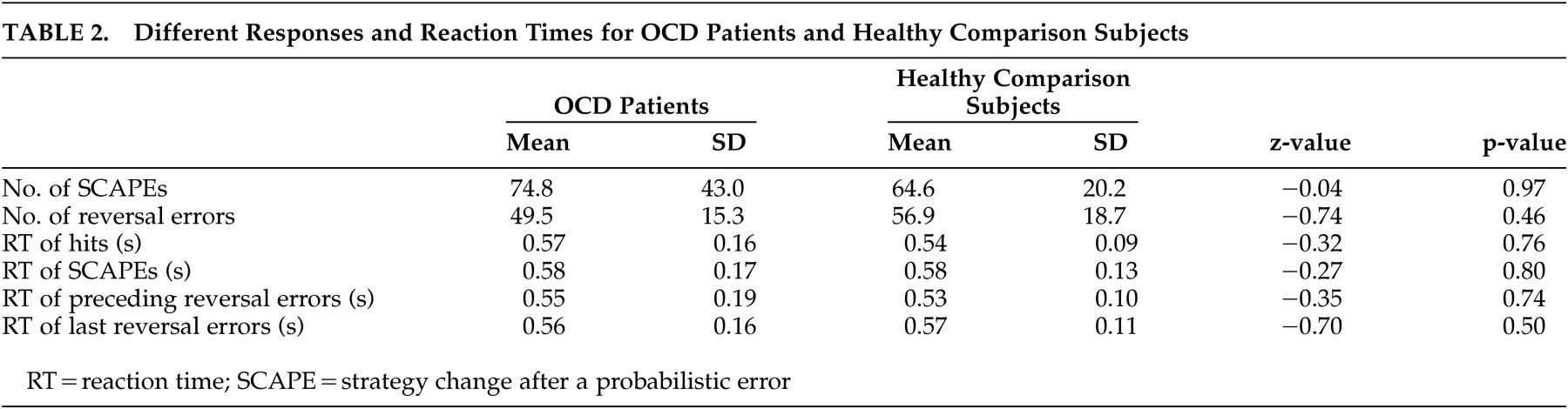
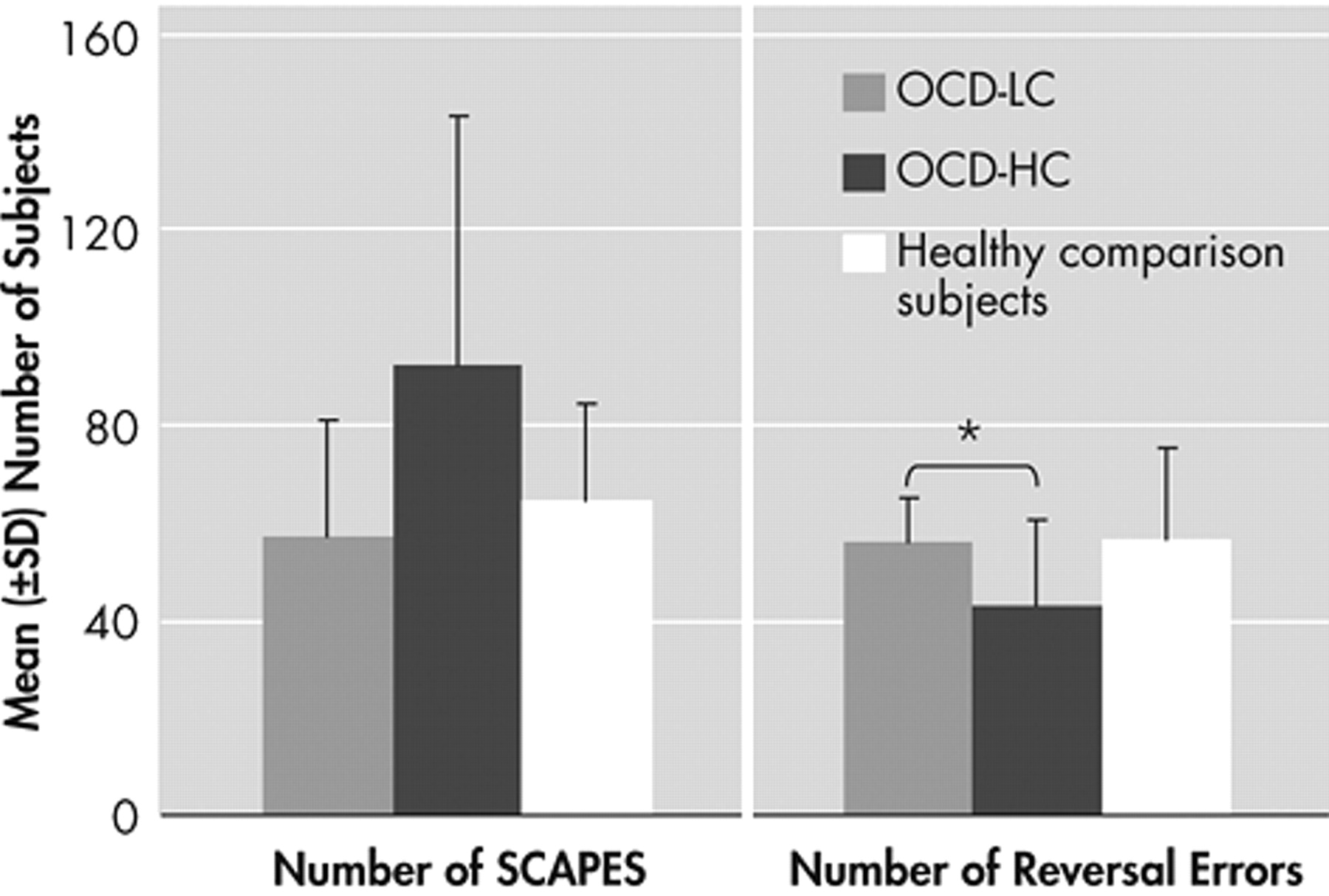
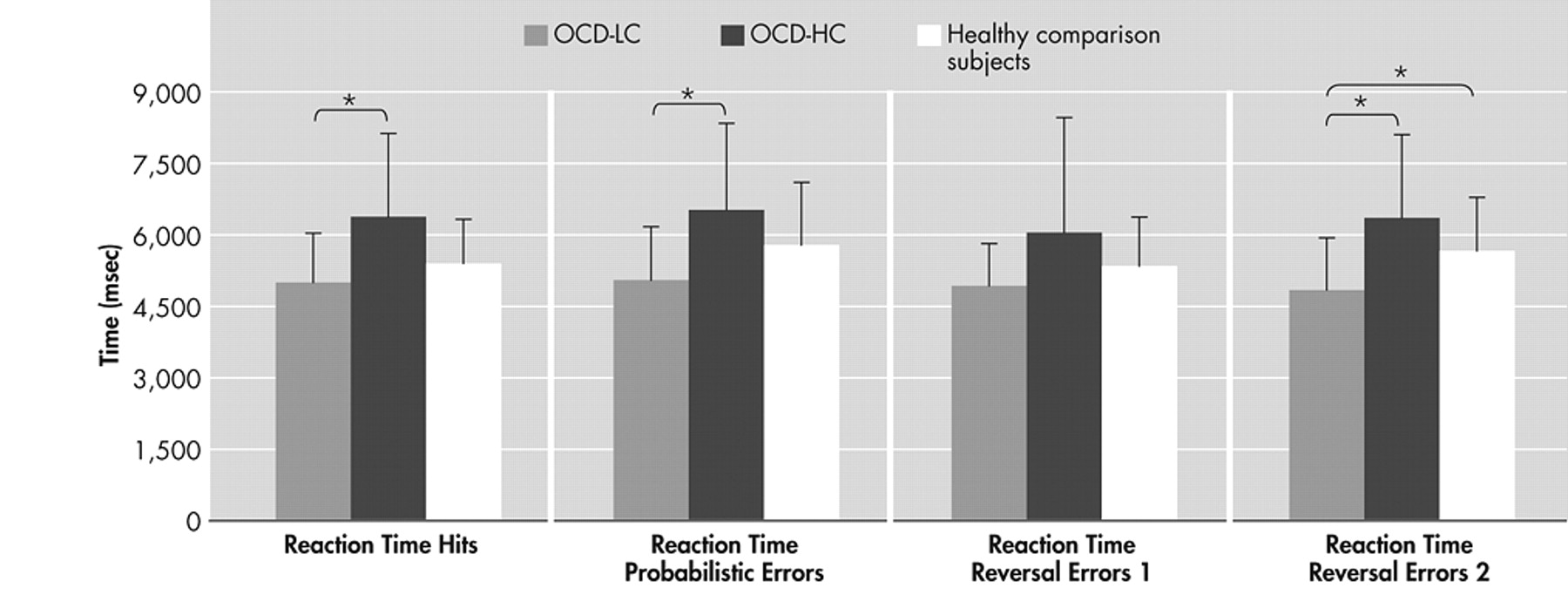
13 The main findings of this study are prolonged reaction times of several behavioral measures of the reversal learning task with increasing severity of compulsions in the patients with OCD. These results are further corroborated by significantly faster reaction times concerning hits, strategy changes after a probabilistic error and last reversal errors of the patients with minor compulsions compared to those with severe compulsions. The positive correlation between severity of compulsions and the different reaction times demonstrates stronger response reluctance with increasing compulsion severity in OCD. Although patients respond correctly, theses reactions are delayed. Considering probabilistic errors, patients with OCD do not show more strategy changes after a probabilistic error than healthy comparison subjects, but it takes them longer to make a decision in a confusing situation. These findings are in line with the results of a study by Maltby et al.,
26 who used a speeded reaction time task with high conflict trials to investigate action-monitoring processes in patients with OCD. Only correctly rejected, high-conflict trials produced excessive activation in fronto-striatal regions. According to Maltby et al.,
26 these findings suggest that correctly rejected, high-conflict trials that require response inhibition might be a better model of compulsive behaviors in OCD than error trials. A recent study by Chamberlain et al.
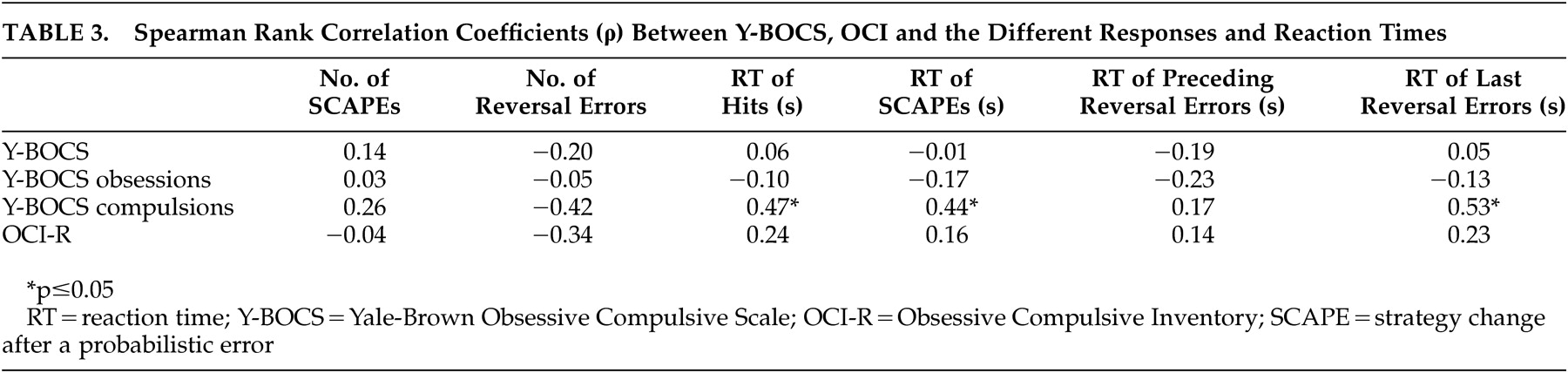
27 also employed a reversal learning task comparing patients with OCD to patients with trichotillomania and healthy comparison subjects. As in our study, the authors did not find any differences concerning the overall number of errors made as well as the number of perseverative errors after reversal. Based on these findings they concluded an intact performance of the patients with OCD. It would be interesting to analyze additional response parameters besides the different numbers of errors and also consider correlation analyses, since in our study the authors found deficits only in association with symptom severity and in consideration of the reaction times. Interestingly, only the reaction time corresponding to the last reversal error, but not to the reversal errors preceding the last reversal error, slows down with increasing symptom severity. On the one hand this argues against a general slowdown of reactions. On the other hand it shows that flexibility and the ability to change a pattern of behavior might be impaired by severe compulsions. Impaired cognitive flexibility and set shifting abilities have been a major finding in neuropsychological as well as functional imaging studies with OCD patients,
28 –
30 thus supporting our results. Interestingly, Cools et al.
17 found an activation of the ventrolateral prefrontal cortex and the ventral striatum concomitant only with the last reversal error. Thus, our finding of a slowdown in reaction time concerning the last reversal error with increasing compulsion severity supports the disturbance of these brain areas at least in patients with distinct OCD compulsions. This interpretation is further corroborated by Remijnse et al.,
31 who for the first time examined patients with OCD using reversal learning in combination with functional imaging. Investigating the specific neural response on reward and affective switching (comparable to the last reversal error in our paradigm), they found evidence for a disturbance of the orbitofrontal-striatal loop in patients with OCD. Their behavioral data cannot be directly compared to our findings since, as in the study by Chamberlain et al.,
27 Remijnse et al. only assessed the number of reversal learning parameters, but did not measure reaction times. Furthermore, they did not distinguish patients according to symptom severity, but mainly included outpatients with mild to moderate symptom severity. Considering the findings by Cools et al.
17 and Remijnse et al.,
31 an association between reversal learning and fronto-striatal dysfunction in OCD seems to be plausible. Nevertheless, deficits in reversal learning are not restricted to OCD, and dysfunctions in associated neurotransmitter systems need to be considered. A study by Gorrindo et al.
32 found reversal learning deficits in pediatric bipolar patients, characterized by higher error rates and a smaller likelihood of meeting the learning criterion. Furthermore, patients with attention-deficit hyperactivity disorder, patients with antisocial behavior, and pediatric patients with autism also displayed deficits in reversal learning.
33 –
35 Reversal learning deficits in these patients were mainly associated with orbitofrontal dysfunction. In patients with mild Parkinson’s disease medication status significantly influenced their performance in a reversal learning task.
36 Patients on a regimen of medication exhibited impaired reversal shifting relative to patients not taking medication, possibly mediated by an overdose of dopaminergic agents in the ventral fronto-striatal system. Thus, dopamine also seems to play a role in reversal learning. Subsequent group comparisons of patients with OCD with minor compulsions to those with severe compulsions and to healthy comparison subjects further emphasize the effect of symptom severity. Differences were found between patients with low compulsion severity and patients with high compulsion severity concerning various reaction times, again including the last reversal error, with the patients with low compulsion severity being faster than the patients with high compulsion severity. Patients with low compulsion severity were also faster in terms of the last reversal errors than healthy comparison subjects. This implies that patients with OCD do not represent a uniform patient group. On the contrary, deficits in fronto-striatal brain areas might only lead to detectable cognitive impairments at a high level of pathology. Interestingly, patients with high compulsion severity made fewer reversal errors than patients with low compulsion severity. As known from a study by Frost and Steketee,
37 patients with OCD have significantly elevated scores on measures of perfectionism, especially the subscales “concern over mistakes” and “doubts about actions.” To prevent mistakes, the more severely disturbed OCD patients might have responded more carefully, resulting in significantly delayed reaction times, but also less mistakes. Reversal learning consists of different cognitive domains and could be considered a kind of alternation learning. So far, few studies have examined alternation learning in OCD, primarily by using the Delayed Alternation Task (DAT) or the Object Alternation Task (OAT). Findings are equivocal. Whereas some studies have found impairments in alternation learning in OCD patients,
38 –
41 the authors
42 and others
43 could not replicate these findings. As the authors have pointed out, these discrepancies might be due to confounding factors such as interpersonal or social cognitive variables or differences between the Object Alternation Task offline and PC version. There may be different reasons for the fact that our findings are restricted to the severity of compulsions. On the one hand, dysfunction of the fronto-striatal system might be more pronounced in patients with predominantly compulsive symptomatology. There is a need for imaging studies examining cerebral correlates of different classes of symptoms. On the other hand, many of the patients reporting compulsive symptoms suffered from washing compulsions. Hence, the authors cannot exclude the possibility that performance in those cases was disturbed by preoccupation with fear of contamination when completing the task. The authors did not find any correlations between symptom severity and the number of SCAPEs or reversal errors. These results suggest that prolonged reaction times serve as a compensating mechanism for deficits of cognitive flexibility. Whereas others have suggested comorbidity as a confounding variable, possibly blurring group differences in alternation learning,
44 the authors did not find support for this hypothesis. Neither depressive symptoms nor general functioning of the OCD patients influenced their performance on the reversal learning task.
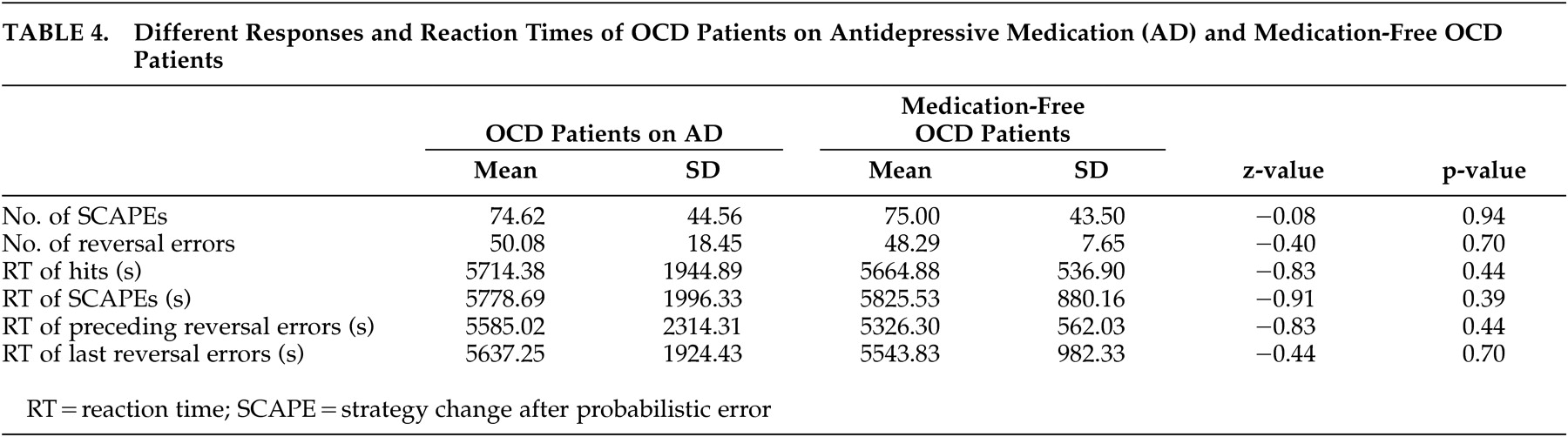
This study has some limitations. Since OCD is supposed to be mediated by a general fronto-striatal dysfunction, the authors intended to include patients of all OCD symptom dimensions. Nevertheless, this heterogeneity could have blurred some effects. As the study by Mataix-Cols and colleagues
45 pointed out, different subgroups of OCD seem to be associated with impairments in different brain networks. Future studies should include a higher number of patients to allow for statistical subgroup analyses concerning different symptom clusters (obsessions versus compulsions, but also different types of obsessions and compulsions) as well as concerning the severity of symptoms. More than one third of our patients were unmedicated; the others were mainly treated with selective serotonin reuptake inhibitors (SSRIs). To exclude an influence of medication on reversal learning, the authors compared the performance of medicated versus unmedicated patients with OCD. In accordance with the finding that SSRIs improve rather than impair behavioral performance, with the exception of long-term memory,
46 the authors could not find any behavioral differences between medicated and unmedicated patients with OCD on the relevant reversal learning task parameters. These findings argue against an influence of medication. Future studies should use functional neuroimaging techniques to determine neural correlates of cognitive functioning. Furthermore, not only symptom severity and subtype, but also possible gender differences, need to be considered, since some studies suggest an influence of gender on neuropsychological performance and clinical characteristics.
In summary, our findings indicate a direct neuropsychological correlate of compulsions resulting in a general slowdown of decision making. On a neural level fronto-limbic impairments seem to subserve this deficit. In support of the proposed neuropathological model the authors found a prolongation in reaction times in OCD patients with increasing severity of compulsions. More generally, the authors suggest the development of neuropsychological assessments in psychiatric populations on the basis of functional neuroimaging findings in healthy comparison subjects.