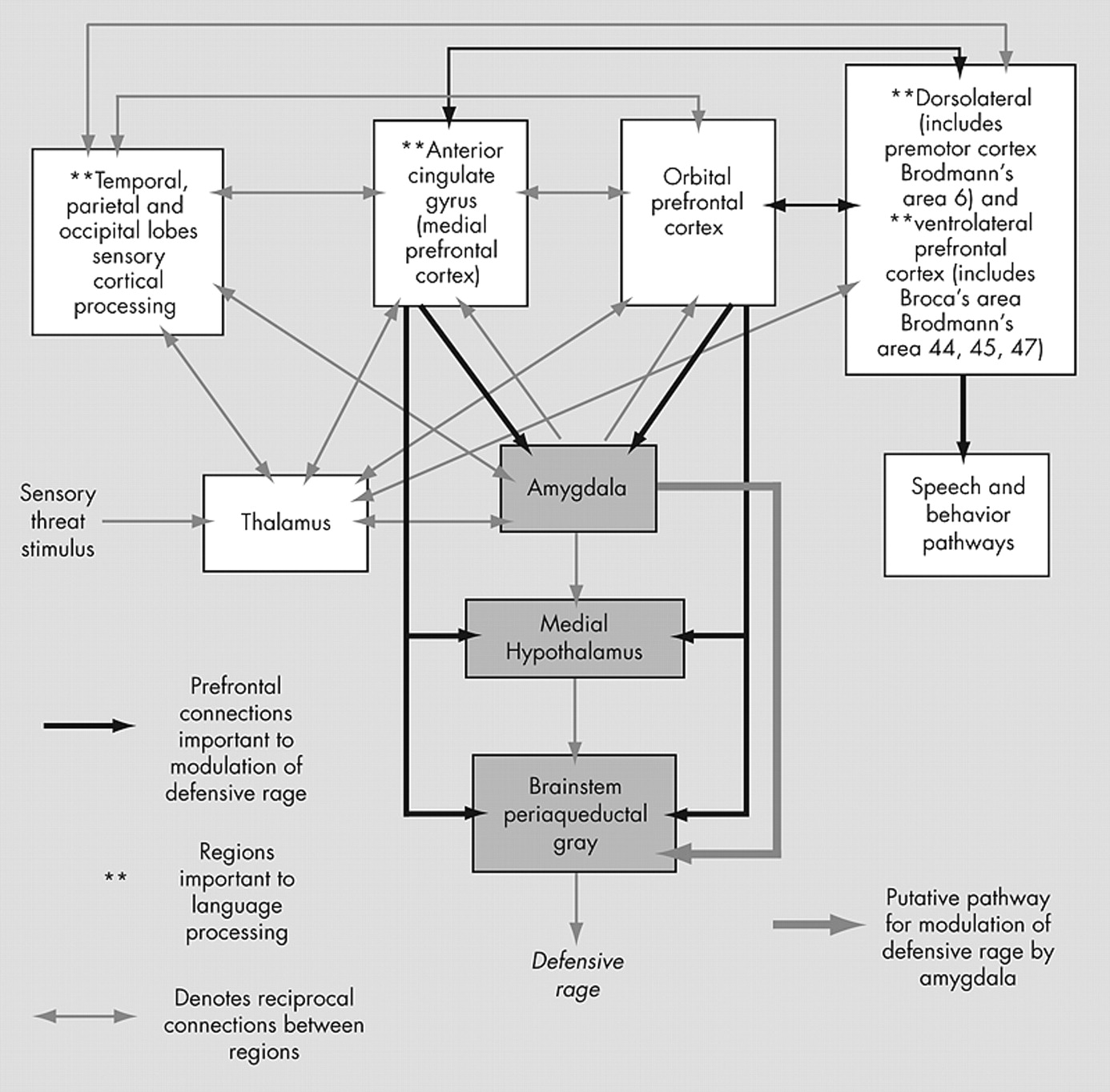
Defensive rage is an automatic, reflexive survival response to overwhelming threat. Its explosiveness resembles human impulsive aggression. The pathway mediating defensive rage involves the medial nucleus of the amygdala, the medial hypothalamus, and the dorsal periaqueductal gray.
13,
24,
29 Independent stimulation of these regions triggers experimental defensive rage in animals, provided that the pathway is intact.
13,
24According to this model, brain regions which inhibit these mediators of defensive rage include the anterior cingulate cortex (ACC)
24 and the orbital prefrontal cortex (OFC),
13 highly confluent frontal regions of the prefrontal cortex. Data supporting this model include that stimulation of the ACC increases latency to aggression in cats.
30Amygdala
While stimulation of the amygdala triggers defensive rage, the amygdala also modulates defensive rage.
13,
24 Amygdala lesions may increase or decrease aggression in humans.
13 Impulsive aggressors commonly report in retrospect that when angry, they “lose control,” neither able to modulate anger nor aggressive impulses. This fits well with Stanford et al.’s
4 proposal that impulsive aggressors may have deficits in physiological arousal and that sudden surges in arousal induce emotional states that are difficult to control. Imaging studies of impulsive aggressors demonstrate abnormalities of the amygdala. In a volumetric structural MRI study, van Elst et al.
11 demonstrated severe atrophy of the amygdala, or lesions involving the amygdala, in a subset of individuals with temporal lobe epilepsy and impulsive aggression, compared to individuals with temporal lobe epilepsy without impulsive aggression. In subjects with borderline personality disorder and impulsive aggression,
10 fluoxetine was associated with a reduction in impulsive aggressive acts and increased metabolism in the medial temporal lobes, including the amygdala (Antonia New, M.D., personal communication, 2006). A separate study of borderline personality disorder and impulsive aggression subjects relative to healthy comparison subjects by New et al.,
31 utilizing positron emission tomography (PET) imaging coregistered with MRI, found differences in amygdala circuitry in borderline personality disorder and impulsive aggression subjects manifested by the loss of ventral-dorsal anatomic specificity in association with functional “disconnection” of the ventral amygdala with the OFC (Brodmann’s areas 11, 12) and Brodmann’s area 47 and other prefrontal regions. In contrast, comparison subjects did demonstrate ventral-dorsal anatomic specificity of the amygdala in association with functional correlations, suggesting “tight coupling” of the right ventral amygdala with the OFC (Brodmann’s areas 11 and 12), Brodmann’s area 47, and Brodmann’s area 44. Aggression scores in borderline personality disorder and impulsive aggression subjects, as measured by the Buss-Durkee Hostility Inventory (BDHI), negatively correlated with metabolic rate in ventral and dorsal regions of the right amygdala.
Zagrodzka et al.
32 found that in cats, unilateral lesions of the central nucleus of the amygdala increased expression of defensive rage. Seigel et al.
33 found that enkephalinergic projections from the central nucleus of the amygdala to the dorsal periaqueductal gray produce potent inhibition of defensive rage via mu receptors in cats, providing a mechanism by which lesions of the amygdala increase aggression.
29 Lesions of excitatory glutamatergic prefrontal cortical neurons, which inhibit the amygdala via synapses with inhibitory interneurons,
27,
34 may also increase aggression.
Accurate identification of emotion in facial expressions and social cues relies on intact amygdala and orbitofrontal cortical functioning.
35 –
37 In a study of subjects with intermittent explosive disorder compared to subjects without, Best et al.
38 found that subjects with the disorder inaccurately identified emotion in pictures of facial expressions, which is consistent with reports of misperception of intent during impulsive aggressive episodes. Intermittent explosive disorder subjects were significantly more prone to label neutral expressions as fear and disgust and demonstrated impaired recognition of facial expressions of surprise and anger. These authors concluded that the bias to interpret neutral expressions as negative, and the inability to recognize cues of impending threat which would inhibit aggression in healthy subjects, might contribute to provocation of aggression in individuals with intermittent explosive disorder.
Affective startle appears to involve frontal-amygdala circuitry.
39 Recently, Hazlett et al.
39 reported that borderline personality disorder patients characterized by impulsivity and aggression show greater-than-normal affective startle eyeblink amplitude during processing of unpleasant words, indicating heightened emotional processing. Yet on self-report, borderline personality disorder patients rated the unpleasant words as less unpleasant than healthy comparison subjects, suggesting that individuals with impulsivity and aggression have deficits in labeling their own emotions. While it is not clear from this study that the borderline personality disorder patients would meet criteria for impulsive aggression, the findings are consistent with abnormalities in emotional control in a population with a high incidence of impulsive aggression. In summary, dysfunction of key regions of the amygdala (or its prefrontal connections with the orbital prefrontal cortex) that modulate arousal and defensive rage, or that contribute to networks involved in the discrimination of emotional cues, may explain the importance of amygdala abnormalities in neuroimaging studies of impulsive aggressors. The complexity of the amygdala, however, precludes a straightforward relationship to emotional control and, by extension, aggression. For example, Kim and Hamonn
40 demonstrated amygdala activation to both negative stimuli (e.g., traffic accidents, vermin, domestic violence, bodily injury) and positive stimuli (celebrations, sporting events, romantic couples) and during regulation of positive emotions, but not during regulation of negative emotions.
Medial Orbital Prefrontal Cortex
It has been proposed that abnormalities of the anterior cingulate cortex (ACC) and orbital prefrontal cortex (OFC) regions of the medial prefrontal cortex, alone or in combination with abnormalities of the amygdala, underlie the hyperarousal/dyscontrol states seen in impulsive aggressors.
9,
10,
13,
14,
38 Blair
13 and Davidson et al.
14 proposed that the ACC and OFC are normally activated during anger arousal via serotonergic mechanisms and exert inhibitory influence over aggressive emotional responding via mechanisms including inhibition of the amygdala, hypothalamus, and brainstem periaqueductal gray. Healthy comparison subjects have been shown to activate the OFC when angry.
13 Studies of impulsive aggressors have found hypoactivation of the ACC and OFC regions of the medial prefrontal cortex.
9,
10,
38 The OFC carries out low-level appraisals of punishment/reward values of behavioral responses (in contrast to complex punishment/reward appraisals performed by lateral prefrontal cortical regions) and of the appropriateness of behaviors in accordance with social cues.
Within the current model of defensive rage, conditions of overwhelming threat may “release” defensive rage from inhibitory modulation. It remains unclear as to how this “release” occurs in humans. One hypothesis consistent with the current model (
Figure 1 ) is that the ACC and OFC regions in impulsive aggressors may lack sufficient levels of activation, with resultant insufficient inhibition of rage responses. New et al.’s
9 findings of blunted OFC (Brodmann’s area 10) and ACC (Brodmann’s area 32) activation in response to a serotonergic stimulus utilizing PET imaging support this hypothesis, as do findings
10 that fluoxetine increased metabolism in the medial OFC, in association with decreased aggression. An alternative hypothesis within this model is that defensive rage may deactivate prefrontal cortical regions that modulate its expression, thus releasing defensive rage responses from prefrontal inhibition. Garcia et al.’s
41 findings that increasing degrees of conditioned fear led to the deactivation of medial prefrontal cortex in mice support this hypothesis.
Lateral Prefrontal Cortex
During the 1990s, growing evidence of neurobiological differences between impulsive and premeditated aggression inspired functional neuroimaging studies of aggressive individuals using a variety of descriptions that share similarities with more recent characterization of impulsive aggression. Many of these studies employed [
18 F]fluorodeoxyglucose (FDG) PET. For example, Volkow et al.
47 compared eight psychiatric patients with “purposeless, repetitive violent behavior” with eight healthy comparison subjects and found significantly lower metabolic rates in bilateral medial temporal and prefrontal cortices. Raine et al.
44 compared nine “affective” murderers, 15 “predatory” murderers, and 41 age- and sex-matched healthy comparison subjects while they performed a Continuous Performance Test. They found that bilateral medial
and lateral prefrontal cortical hypometabolism significantly distinguished “affective” murderers from both “predatory” murderers and healthy comparison subjects. “Affective” murders, compared to both “predatory” murderers and healthy comparison subjects also had higher right subcortical (thalamus, hippocampus, midbrain, amygdala) metabolism and lower right hemisphere prefrontal cortical/subcortical ratios. Raine et al.,
44 studying 41 murderers categorized as “not guilty by reason of insanity,” analyzed 41 healthy comparison subjects and found that “affective” murderers, relative to comparison subjects, had lower right and left medial
and lateral prefrontal hypometabolism, and abnormal subcortical asymmetries (left hemisphere lower than right) of metabolic activity in the thalamus, amygdala, and medial temporal lobe. Goyer et al.
48 studied 17 personality-disordered individuals compared to 43 healthy comparison subjects and found an inverse correlation between a life history of aggressive impulse difficulties and regional metabolism in the
lateral orbital prefrontal cortex, including Brodmann’s area 47.
In considering these findings in the context of their functional significance, it should be noted that variation exists in the classification of Broca’s area. Some authors include Brodmann’s areas 44, 45, and 47,
49,
50 including the pars orbitalis of Brodmann’s area 47,
51 as Broca’s area, while others include Brodmann’s areas 44 and 45 only, but classify Brodmann’s area 47 as a language processing region important to semantic retrieval, decision-making,
52,
53 and syntactic and phonological processing.
50,
53 –
55 Brodmann’s area 47 extends ventromedially from the lateral aspect of the inferior frontal gyrus into the orbitofrontal cortex, including the orbital aspect of the inferior frontal gyrus, and is therefore also referred to as orbitofrontal cortex.
56 Edmund Rolls suggests that Brodmann’s area 47 is a language processing region with connections to contiguous regions of the orbitofrontal cortex (personal communication, 2006). For the purposes of this article, we classify Brodmann’s area 47 as part of Broca’s area. However, for clarity, where studies reviewed in this article find significant changes in Brodmann’s area 47 unaccompanied by changes in Brodmann’s areas 44 or 45, the region will be referred to simply as Brodmann’s area 47.
Davidson et al.
14 proposed that abnormalities of the amygdala, ACC, OFC and “other” regions of the prefrontal cortex with which the OFC is connected constitute the critical nodes of networks that modulate impulsive aggressive outbursts. By extension, we propose that these “other” regions of the prefrontal cortex include the premotor cortex and Broca’s area. Recent neuroimaging studies utilizing more rigorous criteria and nomenclature for impulsive aggression and more detailed structural and functional methods demonstrate hypofunctioning (by functional neuroimaging) or volumetric decreases (by structural MRI) in the inferior frontal gyrus (Brodmann’s areas 44, 45, 47),
9,
10 which overlaps extensively with Broca’s area (Brodmann’s areas 44, 45, 47), and the left premotor cortex.
12New et al.
9 found that blunted activation in bilateral Broca’s area (left Brodmann’s area 45 and right Brodmann’s area 47) accompanied the lack of ACC and OFC activation in response to metachlorophenylpiperazine (m-CPP), a serotonin agonist, with blunted prefrontal response much greater on the left. Hazlett et al.
57 found that borderline personality disorder subjects with greater white matter volume in Brodmann’s areas 44 and 47 had greater irritability-assaultiveness subscale scores on the Buss-Durkee Hostility Inventory (BDHI). They hypothesized that greater white matter volume may be a marker of inefficient white matter processing and/or connections. New et al.’s
10 study of subjects with borderline personality disorder and impulsive aggression demonstrated that fluoxetine treatment of impulsive aggression was associated with changes in Brodmann’s area 47 that correlated with changes in temporal lobe neocortex, including Brodmann’s area 22 (middle temporal gyrus) on the right, and with Brodmann’s areas 20, 21 (superior temporal gyrus), and 22 (middle temporal gyrus) on the left, along with the aforementioned improved activation of the OFC and medial temporal regions including the amygdala. Brodmann’s areas 20, 21, and 22 are language processing regions of temporal lobe neocortex. While not specifically investigating impulsive aggression, Hazlett et al.
57 showed that borderline personality disorder patients, who frequently manifest both impulsivity and aggression, demonstrated marked reductions in anterior cingulate gyrus volume. Woermann et al.
12 found significant decreases in gray matter involving left inferior frontal cortex and left premotor cortex by volumetric MRI in impulsive aggressors. They also found that subjects with temporal lobe epilepsy and impulsive aggression had increases in gray matter volume in the left temporal lobe neocortex, which includes Brodmann’s areas 21 and 22, in contrast to temporal lobe epilepsy subjects without impulsive aggression who demonstrated bilateral gray matter increases in the temporal lobe neocortex without lateralized preponderance. These investigators proposed that the gray matter increases represented gliosis, although this remains to be directly demonstrated.