C ancer is a life-threatening illness and has been recognized as a stressor precipitating posttraumatic stress disorder (PTSD). The incidence of PTSD ranges from 3% in patients with early stage cancer to 35% in patients after treatment.
1 PTSD symptoms cause much distress for cancer patients and are associated with a reduced quality of life.
2 Thus, this issue requires both clinical attention and research.
The cancer experience contains multiple and chronic stressors at each step of diagnosis, treatment, progression, and recurrence. It differs from other types of trauma in that the traumatic stressor never disappears and can cause strong future-oriented anxiety regarding cancer recurrence and death.
3 In addition, the cancer threat originates from within the body; as a result, the patients are never free from the source of their traumatic fears. These unique characteristics of cancer-related trauma suggest that cancer-related PTSD might have a different neurobiological basis, meriting separate study from other types of PTSD. However, to our knowledge, no previous study has focused on this issue.
We previously reported smaller hippocampi
4 and amygdalae
5 in cancer survivors with intrusive recollections, a symptom of PTSD. However, whether these volumetric alterations in cancer patients were related to the entire PTSD entity or only to intrusive recollections remained unclear. With regard to PTSD resulting from other types of traumas, adults with PTSD related to combat exposure or childhood abuse have been repeatedly shown to have smaller hippocampi.
6 These subjects differ from breast cancer survivors in that combat veterans are typically male and childhood abuse victims are traumatized early in life. In addition, a meta-analysis
7 suggested that elderly subjects with severe PTSD tend to have smaller hippocampi. Although the relatively advanced age of patients at the time of cancer onset might contribute to the smaller hippocampi in patients with cancer-related PTSD, the unique characteristics of cancer-related traumas might suggest a different pathophysiology of cancer-related PTSD, compared with other types of PTSD. With regard to amygdalar volume, no reports of significant alterations in patients with PTSD have been made.
The aim of the present study was to examine the hippocampal and amygdalar volumes in breast cancer survivors who had experienced cancer-related PTSD for approximately 1 year since their cancer diagnosis. We hypothesized smaller hippocampal and amygdalar volumes in breast cancer survivors with cancer-related PTSD. Additionally, we examined the possible correlation between regional brain volumes and cancer-related PTSD symptoms.
METHOD
Subjects
This study was approved by the institutional review board and the ethics committee of the National Cancer Center in Japan and was performed after obtaining the written informed consent of all the subjects. All the subjects were part of a data set from a longitudinal prospective study investigating distress and brain volumes in breast cancer survivors. The subjects were recruited from the outpatient clinic of the Division of Breast Surgery, National Cancer Center Hospital East, between 1998 and 2002. A trained psychiatrist (YM) interviewed the subjects using the clinical version of the Structured Clinical Interview for DSM-IV (SCID-I)
8 to diagnose PTSD and other axis I psychiatric illnesses. A sample of 30 subjects assessed by two raters was used to assess the interrater reliability in a preliminary study. The interrater agreement for the diagnosis of PTSD was excellent (κ=1.0). Subjects were excluded if they met any of the following criteria: double cancer or evidence of residual or recurrent cancer detected by an attending oncologist (SI); current comorbid DSM-IV axis I diagnosis at the time of the investigation; psychotropic medication within the previous month; any history of neurologic disorder or traumatic brain injury with loss of consciousness; or cognitive impairment (less than 24 on the Mini-Mental State Examination
9,
10 ). Fifteen cancer survivors with a current (N=5) or past (N=10) history of cancer-related PTSD were eligible and were included in the present study. The subjects in the present study were newly recruited and totally different from those in previous studies.
4,
5As control subjects, 15 breast cancer survivors without any history of psychiatric illness and 15 healthy comparison subjects were individually matched to the subjects with PTSD according to age (±2) and, as closely as possible, to height, years of education, and lifetime alcohol consumption. PTSD symptoms, such as intrusion and avoidance, were assessed using the Impact of Event Scale
11 in all cancer survivors. The Impact of Event Scale is a well-validated, 15-item self-reported questionnaire for measuring intrusive ideation and avoidance and has been used to evaluate cancer-related stress reactions.
The effect sizes for reported differences in hippocampal volume between trauma-exposed subjects with or without PTSD were 1.76
12 for the right, and 1.23
13 and 3.67
12 for the left. For the smallest of these effect sizes, 10 subjects in each group were needed for a two-tailed alpha of 0.05 and a beta of 0.20, suggesting an adequate statistical power for the detection of volumetric differences in the present study.
MRI Image Acquisition and MRI Volumetry
The methods of MRI acquisition and data analysis are described in detail elsewhere.
4,
14 Briefly, MRI scans were performed using a 1.5-T MRI unit (Sigma Scanner; GE Medical Systems, Milwaukee, Wisc.) with three-dimensional spoiled gradient-recalled acquisition of 1.5 mm contiguous sections under the following conditions: field of view=230 mm, matrix=256×256 pixels, repetition time (TR)=25 msec, echo time (TE)=5 msec, and flip angle=45°.
We used the manual tracing method and ANALYZE-PC software, Version 6 (Biomedical Imaging Resource, Mayo Foundation, Rochester, Minn.) to analyze the hippocampal and amygdalar volumes. The volumetric procedure has been described previously.
14 The intraclass correlation coefficients for intrarater variability based on the assessment of 20 subjects and the interrater reliability based on the assessment of 13 subjects were 0.99 and 0.96 for the hippocampus, respectively, and 0.98 and 0.81 for the amygdala, respectively. The intracranial volumes (sums of the gray matter, white matter, and CSF volumes) were calculated from non-normalized segmented images using Statistical Parametric Mapping Software 2 (Welcome Department of Cognitive Neurology, London).
Memory Function
The memory function of the subjects was assessed as a surrogate marker of hippocampal function using the Wechsler Memory Scale—Revised (WMS-R)
15 This test examines both logical and figural memory, producing immediate and delayed memory scores for each parameter. We also calculated the percentage of retention, defined as delayed/immediate×100.
Statistical Analyses
The normalized volume values, defined as the absolute volume/intracranial volume, were analyzed by repeated-measures analyses of covariance (ANCOVA) with side as the repeated-measures (within-group) factor and age and alcohol consumption as covariates among the groups. A chi-square test, two-tailed Student’s t test, and one-way analysis of variance (ANOVA) with post hoc Tukey’s honestly significant difference tests were used to compare the subjects’ characteristics. The indexes of the WMS-R and absolute regional brain volumes were also compared using an ANOVA. Additionally, we examined a partial correlation between the Impact of Event Scale subscores and volumetric variables, controlled for age and alcohol consumption, in the PTSD group (two-tailed). A p value of less than 0.05 was considered statistically significant. All data analyses were performed using statistical software SPSS version 12.0 J for Windows (SPSS Japan Institute Inc., Tokyo).
RESULTS
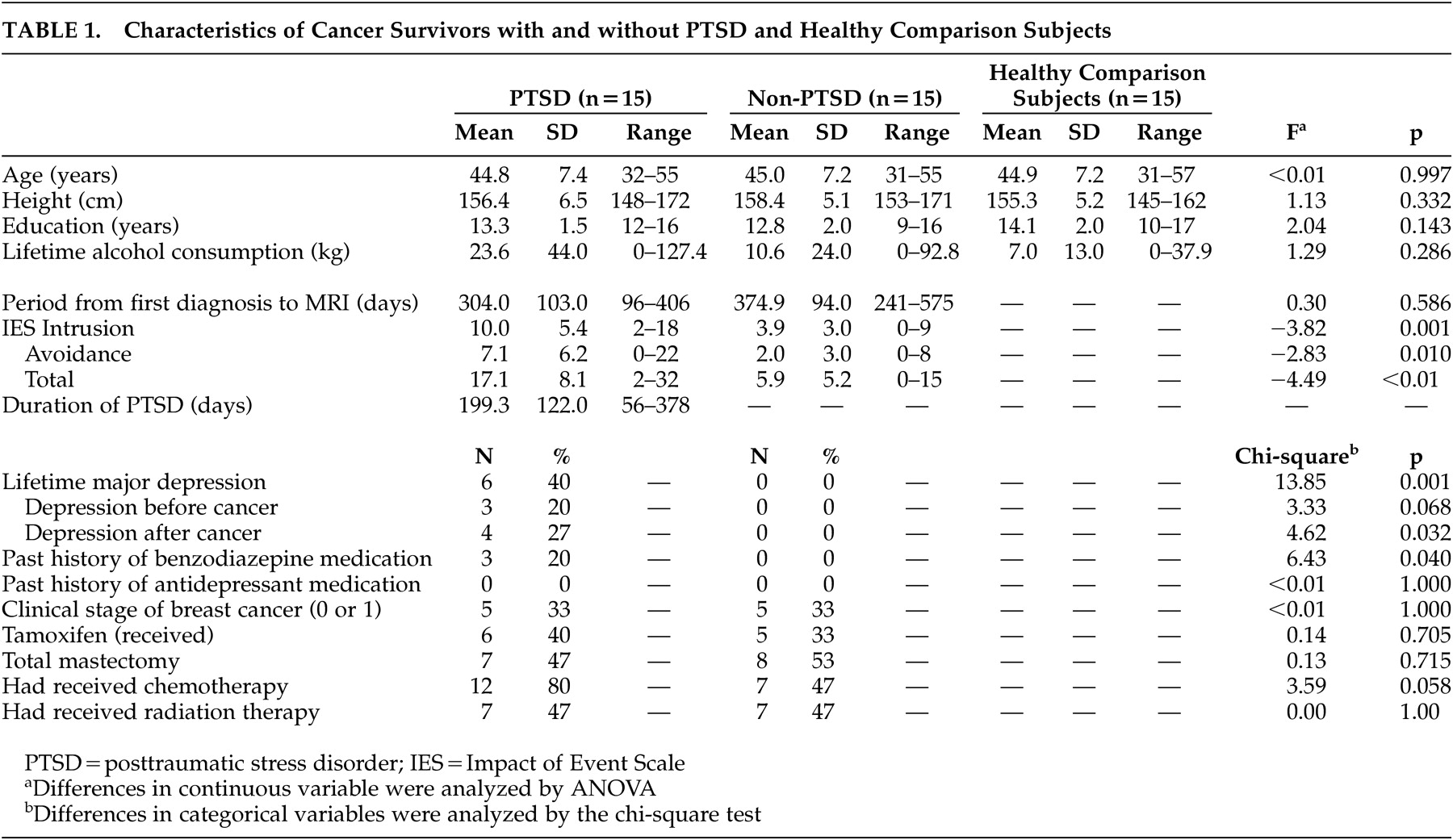
No statistical differences in demographics (age, height, education, and lifetime alcohol consumption) were seen among the three groups (
Table 1 ). The lifetime history of major depression and the past history of benzodiazepine medication were significantly different between the PTSD and non-PTSD groups, but other medical background characteristics, including the use of adjuvant chemotherapy or endocrinological treatments, were not significantly different. As expected, the PTSD subjects had significantly higher intrusion, avoidance, and total Impact of Event Scale scores compared with the non-PTSD subjects.
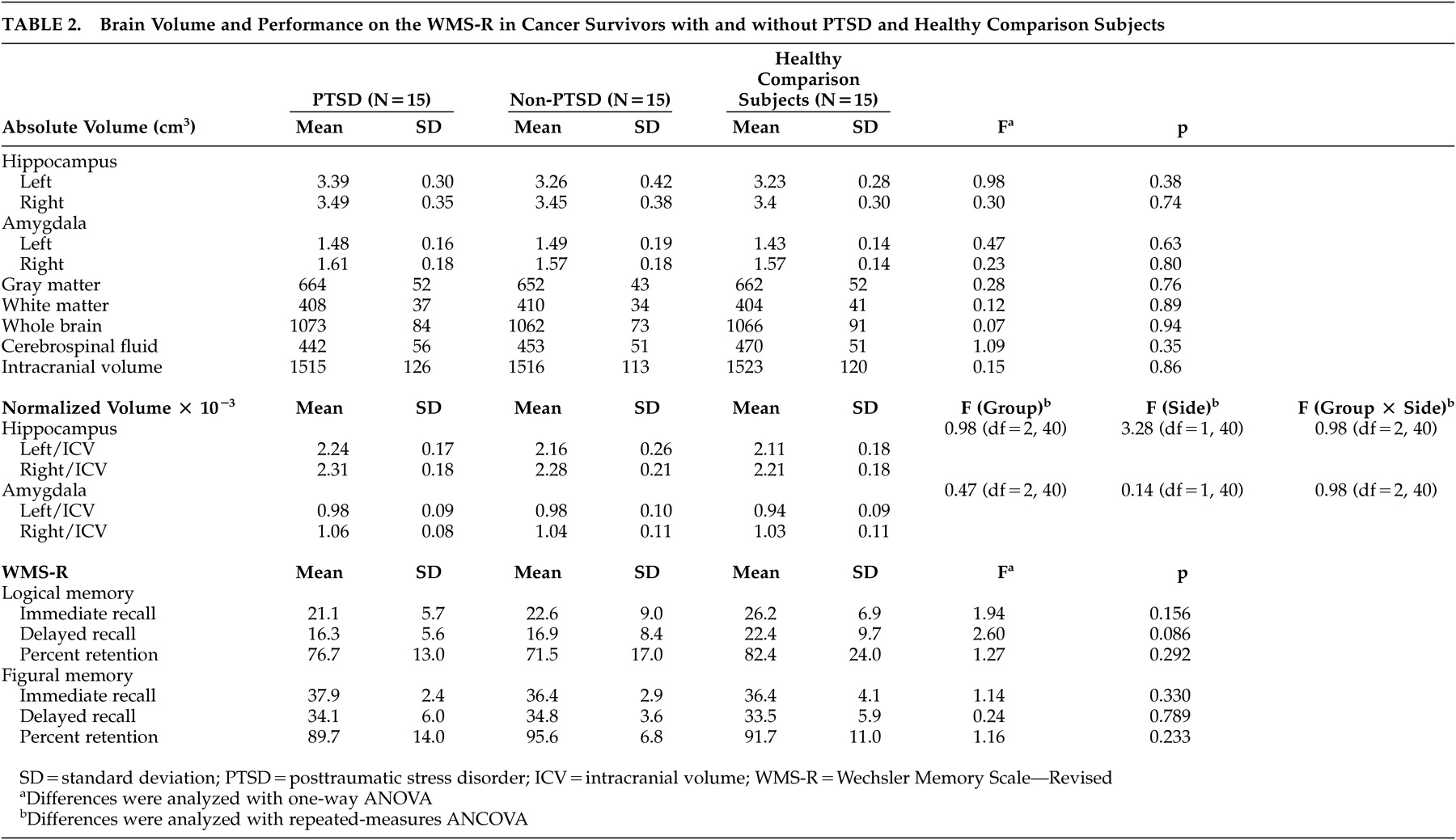
A repeated-measures ANCOVA showed no significant main effect among the three groups according to side, and no significant group-by-side interactions for the normalized hippocampal and amygdalar volumes (
Table 2 ). We confirmed the absence of associations between these volumes and possible confounding background characteristics with trend-level differences between the groups, as shown in
Table 1 (p<0.10). In addition, a repeated-measures ANCOVA after including these factors as covariates did not show any significant main (group) or interaction effects on the hippocampal (main: p=0.44, interaction: p=0.13) or amygdalar (main: p=0.76, interaction: p=0.34) volumes. None of the WMS-R indexes differed significantly among the three groups (
Table 2 ).
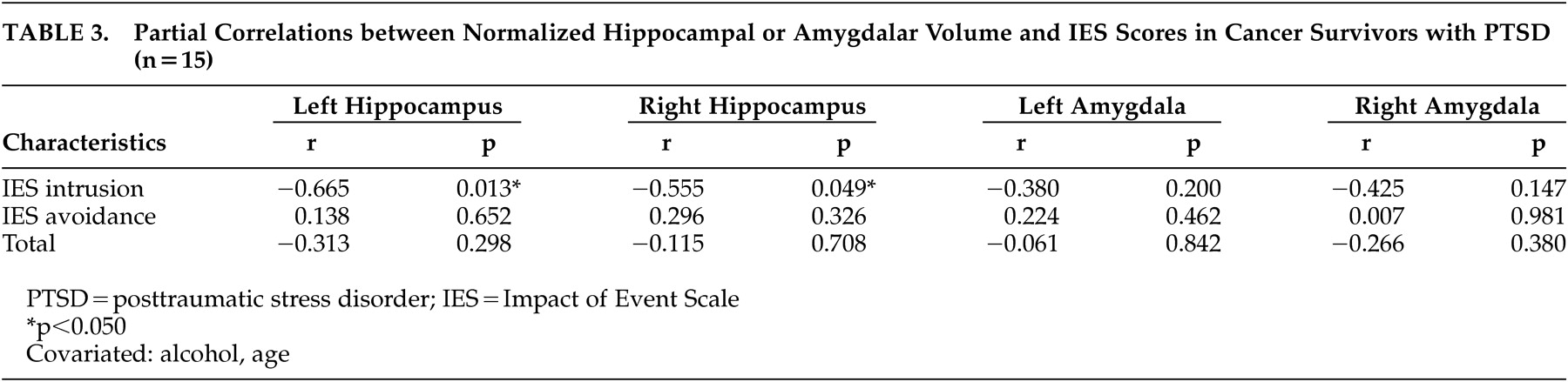
Additional analyses examining the relationship between PTSD symptoms and volumetric variables revealed an inverse association between the left or right hippocampal volume and the intrusion subscale score, but not the avoidance subscale score, of the Impact of Event Scale in the PTSD group (
Table 3 ). No significant correlations were found between the amygdalar volume and the Impact of Event Scale subscale scores.
We also examined the correlations between each WMS-R index, as surrogate markers for hippocampal function, and the Impact of Event Scale scores in the PTSD group, but did not find any significant correlations (data not shown).
DISCUSSION
This is the first study examining the hippocampal and amygdalar volumes in subjects with cancer-related PTSD compared with those not only in healthy comparison subjects but also to those in matched non-PTSD cancer survivors. Cancer-related PTSD was not associated with either hippocampal or amygdalar volume at approximately 1 year after cancer diagnosis. Furthermore, memory functioning in the PTSD group did not differ significantly from that in the non-PTSD or healthy groups.
The lack of association between the hippocampal volume and PTSD in the present study can be explained as follows. First, the duration of PTSD in this study was relatively short. Second, the severity of the PTSD might not be sufficient to alter the hippocampal volume. Kitayama et al.
6 summarized previous studies demonstrating that smaller hippocampi were typically observed in adults with long-standing and severe PTSD. Bonne et al.
16 reported that the hippocampal volumes of subjects with moderate PTSD did not differ from those without PTSD at 1 week or 6 months. Moreover, smaller hippocampi were not related to PTSD at 16 months after trauma
17 ; meanwhile, the follow-up period of the present study was about 12 months. Although one positive study
18 showed smaller right hippocampi in PTSD subjects who had experienced trauma 158 days on average prior to the study, it did not set up the traumatized control group. Third, the distinctive features of the cancer experience as a traumatic event might contribute to the present results. Cancer-related PTSD might have a different neurological basis from that of other PTSDs because of its unique characteristics.
The present study did not demonstrate the presence of smaller hippocampi in breast cancer survivors with PTSD at about 1 year after the first experience of their traumas. To date, various authors have discussed whether a smaller hippocampal volume may be a predisposing factor in the development of PTSD
19,
20 or the consequence of traumatic events and subsequent PTSD.
20,
21 The present negative findings, considering the relatively short period since the onset of trauma, may indicate that a smaller hippocampal volume is not likely to predispose cancer subjects to developing PTSD. However, further longitudinal studies investigating hippocampal volume in cancer-related PTSD are needed to form a definite conclusion.
Although cancer-related PTSD was not associated with hippocampal volume, additional analyses revealed an inverse association between intrusive symptoms and hippocampal volume, which is in line with our previous study reporting smaller hippocampi in cancer survivors with intrusive symptoms.
4 These results may suggest that intrusive symptoms, rather than cancer-related PTSD, are associated with hippocampal volume. Moreover, this association was significant in the PTSD group but not in the other groups, suggesting that intrusions in subjects with PTSD might be pathophysiologically different from those in non-PTSD subjects. This conclusion, however, remains speculative.
With regard to the amygdala, no previous reports have described volumetric alterations in patients with PTSD. However, we previously reported smaller amygdalae in cancer survivors who experienced intrusive recollections more than 3 years after their surgeries.
5 Nevertheless, the present study did not show any association between amygdalar volume and cancer-related PTSD 1 year after trauma. These results may indicate that a longer duration of PTSD or intrusive symptoms is needed to cause volumetric alterations in the amygdala, but this topic also requires further longitudinal investigations.
The inclusion of subjects with a past history of PTSD and the lack of information on PTSD severity are two limitations of the present study. Despite these limitations, our study also has several strengths. Our study was a well-matched control study with adequate statistical power, and all the subjects were right-handed women without any current psychiatric comorbidity. Moreover, we used both traumatized and nontraumatized comparison subjects, whereas most previous studies used one or the other.
In conclusion, hippocampal volume is not associated with cancer-related PTSD but may be associated with intrusive symptoms in cancer survivors. A future study focusing on intrusive symptoms, rather than full PTSD, is needed to resolve the neurobiology of distress in cancer survivors.
Acknowledgments
This study was supported in part by a third-term Comprehensive Research for Cancer Control from the Japanese Ministry of Health, Labour and Welfare and a Grant-in-Aid for Scientific Research (KAKENHI WAKATE B-16790711) from the Japanese Ministry of Education, Culture, Sports, Science and Technology. We would like to thank Prof. Toru Nishikawa for his thoughtfulness, Dr. Noriyuki Kitayama for his valuable advice, and Ms. Nobue Taguchi, Ms. Yukiko Kozaki, and Ms. Yuko Kojima for their research assistance.