N eurodevelopmental disturbances have been proposed as a crucial factor in the etiology of schizophrenia.
1 Brain-derived neurotrophic factor (BDNF) belongs to the neurotrophic factor family, plays a critical role in neuronal growth, survival, and differentiation of the meso-limbic dopaminergic system,
2,
3 and regulates normal expression of the dopamine D3 receptor (D3R).
4 Changes in BDNF protein levels, its receptor TrkB, or its mRNA expression in the hippocampus and cortical areas have been reported in patients with schizophrenia,
5 –
7 suggesting that alterations in
BDNF gene expression may be implicated in the pathogenesis of schizophrenia. Thus, the
BDNF gene appears to be a suitable candidate for genetic association study of schizophrenia.
The
Val66Met (rs6265) is a functional polymorphism in the coding region of the
BDNF gene that results in a valine (Val) to methionine (Met) substitution in the 5′ prodomain of the human BDNF protein.
8 This polymorphism is of great interest because of its impact on intracellular trafficking, activity-dependent secretion of BDNF, and human memory-related hippocampal activity.
9 A previous case-control study reported a trend association between
Val66Met genotypes and schizophrenia among Han Chinese.
10 Recently, such an association between the Val allele and schizophrenia spectrum disorders was found to be significant in a Scottish case-control sample
11 and in another family-based association study.
12 However, most other studies reported no association between this polymorphism and schizophrenia in Asian
13 –
17 and Caucasian individuals.
18 –
20 Similarly, several meta-analyses
13 –
15,
21 (including the largest published so far
22 ) did not find any association between this polymorphism and schizophrenia. Interpretation of these disparate results is complicated by three issues. In individual studies, either sample size was insufficient, patient populations were heterogeneous in clinical symptoms or genetic background, or a means to correlate the Val66Met phenotype with specific and quantifiable clinical symptoms was lacking. To address these concerns, we recruited larger patient and comparison populations, subdivided patient populations according to age of onset or family history for statistical analyses, and examined the relationship between BDNF genotype and specific psychopathological symptoms beyond a global diagnosis of schizophrenia.
It has been proposed that insufficient dopaminergic transmission in the prefrontal cortex results in negative symptoms in schizophrenia.
23 Guillin et al.
4 identified BDNF as an inductor of the synthesis of D3R, which is considered to have autoreceptor properties modulating the synthesis and release of dopamine.
24 Recent evidence suggests that higher levels of D3R mRNA correlate with negative symptoms of schizophrenia,
24 and that preferential prefrontal D3 receptor antagonism may explain the benefits of atypical antipsychotics in alleviating negative symptoms.
25 Taken together, these studies suggest a link between the
BDNF gene and the negative symptoms of schizophrenia, owing to the ability of BDNF to control of D3R expression. This hypothesis seems to be supported by a previous family-based study.
26 Fanous et al.
26 reported a modest association between negative symptoms and a dinucleotide repeat in the
BDNF gene, even though the effect of the dinucleotide repeat on
BDNF gene function is still unclear. More recent studies suggested that the
BDNF Met/Met neurons showed defective secretion of BDNF
27 and that the Met allele possibly influenced the expression of BDNF mRNA in the prefrontal cortex.
28 Further investigation is therefore required to establish whether the
BDNF Val66Met genotype has a differential effect on the negative symptoms of schizophrenia.
Since BDNF plays a critical role in the neurodevelopment hypothesis of schizophrenia,
1 and since BDNF controls the normal expression of D3R,
4 we hypothesized that the
BDNF Val66Met polymorphism would influence not only the susceptibility to schizophrenia, but also its negative symptoms. To test this hypothesis, we investigated the association between the
BDNF Val66Met polymorphism and schizophrenia by comparing the frequency of this polymorphism in patients with schizophrenia and healthy comparison subjects. In addition, it has been postulated that focusing the investigation on specific subclinical phenotypes may increase the strength to detect genes involved in complex disorders, for example, schizophrenia.
29 Thus, to reduce the clinical heterogeneity, this study further examined the patient subgroups according to clinical variables, including age of onset
30 and family history.
29 We subsequently investigated the association between the
BDNF Val66Met genotypes and the severity of negative symptoms by assessing initial psychopathology in a subset of patients who were drug-free or drug-naive.
METHODS
Participants
This study was conducted at the inpatient and outpatient units of the Tri-Service General Hospital, a medical teaching hospital belonging to the National Defense Medical Center in Taipei, Taiwan. The protocol was approved by the Institutional Review Board for the Protection of Human Subjects. After complete description of the study procedure to the subjects, written informed consent was obtained according to the Institution’s review board guidelines. To minimize the effect of ethnic differences in gene frequencies, all subjects participated in this study were from the Han Chinese population in northern Taiwan. All the participants were unrelated, born and living in Taiwan, and all of their biological grandparents were of Han Chinese ancestry. Individuals with a history of substance dependence, severe medical illness, organic brain disease, or any concomitant major psychiatric disorders were excluded from this study.
The patient group consisted of 251 patients with schizophrenia who were recruited from clinical settings. Each patient was initially evaluated by an experienced attending psychiatrist (SYH) and then interviewed by a well-trained psychologist using the Chinese Version of the Modified Schedule for Affective Disorders and Schizophrenia—Lifetime Version (SADS-L)
31 to reach a DSM-IV diagnosis. The interobserver reliability (
k values) of the SADS-L are as follows: schizophrenia, 0.95; major depression, 0.79; bipolar disorder, 0.7; anxiety disorder, 0.86; and substance abuse and dependence, 0.82.
32 The age at the first psychotic episode, as recorded during the clinical interview or in the medical records, was used as the age of onset. Onset of schizophrenia was characterized as early or late based on whether the first psychotic episode occurred before or after the age of 28, respectively. We chose this cutoff because it was a validated threshold used to define the age of onset in the search for schizophrenia susceptibility factors.
30 Family history indicated a report of at least one first-degree relative who had been in contact with psychiatric services and given a schizophrenia diagnosis. In order to reduce the clinical heterogeneity of schizophrenia,
29 patients were further classified into four homogenous clinical subgroups: schizophrenia with family history, schizophrenia without family history, early-onset schizophrenia, and late-onset schizophrenia.
The comparison group included 284 healthy volunteers recruited from the community. The modified Chinese Version of SADS-L
31 was used to exclude individuals with psychiatric conditions. Comparison subjects were considered free of past or present major or minor mental illnesses (affective disorder, schizophrenia, anxiety disorder, personality disorder, substance use disorders, etc.). Also, comparison subjects had no history of psychiatric disorder in their first-degree relatives.
Psychopathological Assessment
An experienced psychiatrist evaluated all hospitalized patients using the Positive and Negative Syndrome Scale (PANSS)
33 on the day of admission. Patients (n=125) having a minimum baseline PANSS score of 70 and being drug-naive or drug-free for at least 1 month before hospitalization were selected for further analysis, because antipsychotic drugs may influence psychopathology status as well as brain BDNF concentrations and mRNA expression, giving rise to confounding effects.
34Genotyping
Whole blood was drawn from the subjects’ peripheral veins using Vacutainer tubes containing ethylene-diaminetetracetic acid (EDTA). Genomic DNA was extracted from the blood leukocytes using standard methods. The coding variant of G196A (Valine-66-Methionine, rs6265) in the
BDNF gene was detected using the modified polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) methods described by Neves-Pereira and colleagues.
8 All PCR-RFLP experiments were performed in a PerkinElmer 9700 thermal cycler (Boston, Mass.). Thereafter, the PCR products were digested using the
Nla III restriction enzyme. During RFLP analysis, the
Val allele of the
BDNF gene remains intact and is 244-base pairs long, while the
Met allele is cut into two DNA fragments of 167 and 77 base pairs. Genotype data were read by an experimenter blinded to the case status.
All genotyping results were further confirmed by using the bidirectionally direct sequencing technique (3730 DNA Analyzer, Applied Biosystems, Foster City, Calif.) with the primers 5′-TTGGTTGCATGAAGGCTGCCC-3′ and 5′-TCGGCACTGGGAGTTCCAATG-3′. A BigDye terminator v3.1 Cycle Sequencing kit (Applied Biosystems) was used for bidirectionally direct sequencing according to the manufacturer’s instructions. The genotyping error rate for the PCR-RFLP method and the direct sequencing technique was less than 0.005 (as assessed by replicate genotyping of each sample).
Statistical Analyses
The independent-samples t test was employed to determine the difference in mean age between patients with schizophrenia and healthy comparison subjects. Pearson’s χ
2 test was used to compare gender difference between the patient group and the comparison group. The Fisher’s exact test was substituted for the Pearson’s χ
2 test when the expected sample sizes were <5. Genotypic distribution was compared to the predicted value from the Hardy-Weinberg equilibrium. The frequencies of genotype and allele were also compared between patients and comparison subjects using the Pearson’s χ
2 analysis. One-way analysis of variance (ANOVA) was used for comparison of global, positive, negative, and general symptoms scores of the PANSS among three
BDNF Val66Met genotype groups. A multiple logistical regression analysis was also applied to correct the effects of possible covariates such as age and gender. SPSS (version 13.0, SPSS, Taipei, Taiwan) statistical software was used for all analyses, and a probability value p<0.05 was considered statistically significant. Power analysis was performed with the use of G-power.
35 All tests were two-tailed; alpha was set at 0.05.
RESULTS
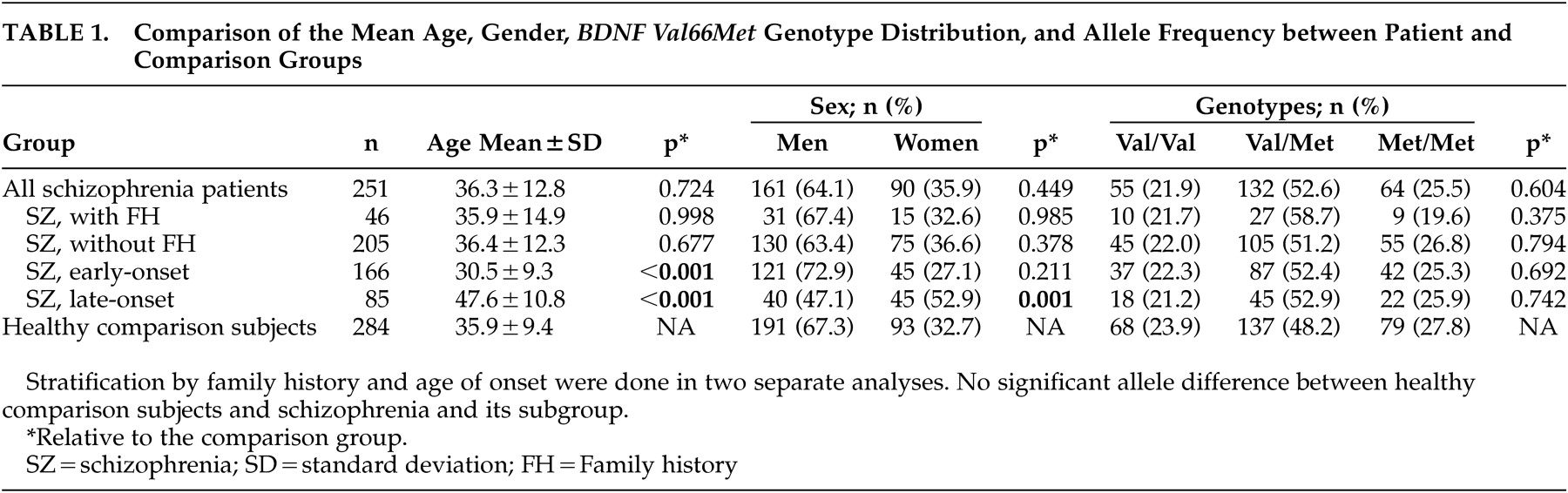
In the total sample, there were no significant age and gender differences between patients and comparison subjects (
Table 1 ). However, patients with early-onset schizophrenia were younger than comparison subjects, while patients with late-onset schizophrenia were older than comparison subjects. Patients with a late onset also had a higher proportion of women relative to comparison subjects. The
BDNF Val66Met genotype distributions were in Hardy-Weinberg equilibrium in both the patient and comparison groups. There were no significantly different
BDNF Val66Met genotype and allele frequencies between schizophrenia patients and controls, and between the four homogenous patient subgroups and controls (
Table 1 ). Genotype and allele comparisons between patients with or without family history as well as comparisons between patients with an early or late onset also did not reveal any statistically significant difference. Also, using multiple logistical regressions to correct for age and gender, there was still no association between the
BDNF polymorphism and schizophrenia or its clinical subgroups.
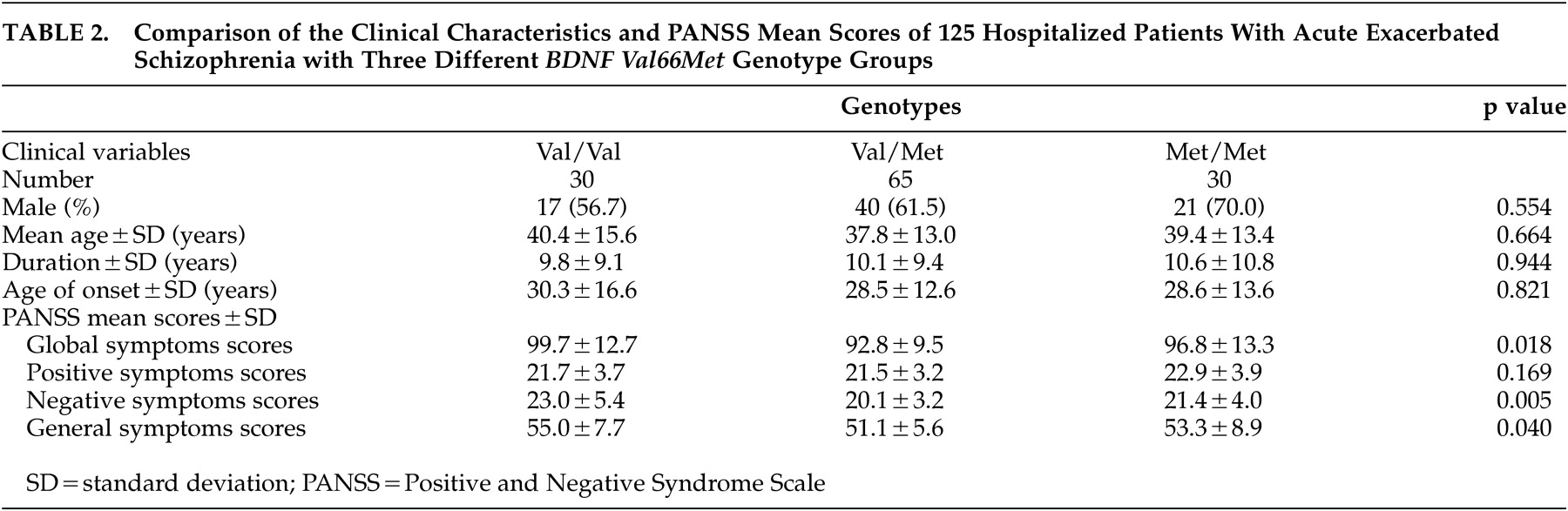
In the 125 hospitalized patients with acute exacerbated schizophrenia, the mean PANSS global, positive, negative, and general symptoms scores were 95.4 (SD=11.6), 21.9 (SD=3.5), 21.1 (SD=4.2), and 52.6 (SD=7.2), respectively. There were significant differences in mean PANSS global (p=0.018), general (p=0.04), and negative (p=0.005) symptoms scores between three genotype groups (
Table 2 ).
The present total sample had a power of 0.53 to detect a small effect, and 0.99 to detect a medium or large effect when comparing genotypes. Comparing alleles, the power was 0.91 to detect a small effect, and 0.99 to detect a medium or large effect. The statistical power was considerably lower when the total patient population was divided into subgroups.
DISCUSSION
A major finding in our study is the lack of significant differences in
BDNF Val66Met allele and genotype frequencies between patients with schizophrenia and comparison subjects. The
Met66 allele frequency in the healthy comparison subjects (0.52) was similar to that found in earlier studies involving Han Chinese (0.43–0.52),
10,
16 but was notably different from that found in Caucasian comparison populations (0.16–0.22).
11,
18 –
20,
36 Our study failed to replicate the association reported in two recent Caucasian studies,
11,
12 which used individuals with schizophrenia spectrum disorders. It is possible that any effect of the
BDNF Val66Met polymorphism on the genetic risk for schizophrenia was too small to be detectable, necessitating the recruitment of a larger study sample to provide the requisite statistical power to determine whether such an association exists. However, recent case-control association studies having quite strong statistical power still did not identify any association in Caucasians
18 and Asians.
14,
16,
17 Another possible explanation is that the homogenous group of patients with schizophrenia examined in the present study, and substantial variations in
Met66 allele frequencies between Han Chinese and Caucasian individuals, contribute to the contradictory result. Compared with a previously published study also involving a Han Chinese population from Taiwan and reporting a higher frequency of the
BDNF Val/Val genotype in patients with schizophrenia (p=0.055) than in comparison subjects,
10 the larger sample size in this study made our results more reliable. Altogether, the lack of association reported here is in agreement with the largest meta-analysis published so far
22 and most other case-control studies, which did not detect any
BDNF Val66Met association with schizophrenia in Caucasian,
18 –
20,
36 Japanese,
17 or Han Chinese populations.
14,
16Schizophrenia is heterogeneous in its expression, possibly owing to the heterogeneity of underlying etiological factors. In order to identify these factors, one needs to define consistent clinical subgroups and relevant phenotypic subtypes. Because age of onset and family history have been considered relevant phenotypic markers in schizophrenia,
29 the patient group was further stratified according to both age of onset and family history. Still, no association between
BDNF Val66Met polymorphism and these more homogeneous subgroups was found. Our results are in agreement with those from other research groups who did not find any association when analyzing family history
20 and age of onset.
19,
20Despite the lack of association between the
Val66Met variants and schizophrenia in the present analysis, it could not be ruled out that other
BDNF polymorphisms are involved in the pathogenesis of schizophrenia. For example, a strong association with schizophrenia has been demonstrated for the C270T polymorphism in the 5′-noncoding region of the
BDNF gene,
37 even though subsequent association studies yielded variable results. Furthermore, the most recent and largest case-control association study indicated that although single-locus analysis of
Val66Met polymorphism provided no evidence for an association with schizophrenia, haplotype analysis of the marker rs988748-(GT)
n -
Val66Met showed a significant association, particularly in a subgroup of patients with a lifetime history of depressive symptoms.
18 Thus, further association studies investigating the contribution of additional
BDNF polymorphisms in schizophrenia are required to clarify the relationship between the
BDNF gene and schizophrenia.
Genetic studies of psychiatric illness have reported a number of modifier genes, which may affect clinical features associated with illness, but without altering the liability to the illness itself. Therefore, investigations focusing on clinical features may help detect such genes.
29 In our study, there were associations between the
BDNF Val66Met polymorphism and total, negative, and general PANSS symptoms scores in acutely exacerbated hospitalized patients with schizophrenia, who were drug-naive or drug-free for month or more. These findings suggest that
Val66Met genotypes may influence the severity of psychopathology in schizophrenia, and negative symptoms in particular. These results are also partially in accordance with the hypothesis that the
BDNF Met/Met genotype is associated with a defective BDNF secretion, resulting in decreased D3R expression and fewer negative symptoms, because
BDNF Val/Val carriers would then be expected to display more negative symptoms. However, there was no linear association between the number of Val alleles and negative symptom scores. Val homozygotes displayed the highest negative symptom scores and Val/Met heterozygotes displayed the lowest, whereas Met homozygotes displayed symptom scores in between. This pattern is suggestive of molecular heterosis.
38We hypothesized that the Val66Met genotype might affect psychopathological symptoms of schizophrenia via effects of BDNF on expression of D3R. Two other studies have reported a link between D3R expression and schizophrenia symptoms in agreement with our results summarized in
Table 2 . Vogel et al.
24 reported that increased D3R expression in peripheral lymphocytes of patients with schizophrenia was associated with negative schizophrenia symptoms, and hypothesized that this was due to alteration of the D2R to D3R ratio. Kwak et al.
39 found that, among drug-free and drug-naive patients with schizophrenia, those with higher D3R mRNA levels in circulating lymphocytes had significantly higher scores on the Brief Psychiatric Rating Scale.
To our knowledge, this is the first study that examines the association between the
BDNF Val66Met polymorphism and psychopathologic symptoms in a moderately sized medication-free subset of patients with schizophrenia. Notably, a recent study suggested that the
Val/Val genotype was associated with more positive symptoms compared to Met carriers in chronic hospitalized patients who were treated with long-term antipsychotic drugs.
40 Failure to avoid confounding effects introduced by antipsychotic drugs may contribute to the differences between our study and the others previously mentioned.
The PANSS score differences between the three BDNF Val66Met genotypes were generally small, and may not be of significant clinical importance. However, given previous molecular genetic research analyzing complex traits, the low magnitude of the present results are of an expected order for the contribution of a single gene variant. If real, the association between the BDNF gene and psychopathology symptoms may be an effect by the investigated polymorphism itself, or another gene variant linked to the Val66Met polymorphism. Still, it is possible that the present results did occur by chance. Therefore, this finding should be considered as preliminary pending replication in new larger samples with medication-free acutely exacerbated patients with schizophrenia.
The present study has some limitations. The power to detect a small effect in genotype distributions was 0.53. Therefore, the finding that the BDNF Val66Met genotypes do not increase the risk for schizophrenia in Han Chinese should be interpreted cautiously. Furthermore, subdividing the patient population according to family history and age of onset resulted in a loss of statistical power because of the limited sample size. Another limitation is that we evaluated psychopathology at a single point in time before treatment. Long-term psychopathology may not be reflected by a single point assessment because psychopathology may vary between different episodes and during the course of illness in each patient. Further studies using ratings based on lifetime psychopathology are needed to reexamine the effect of BDNF Val66Met polymorphism in relation to a longer course of schizophrenia.
CONCLUSION
The results reported herein showed no evidence for a major role of the BDNF Val66Met polymorphism in the predisposition to schizophrenia or its clinical subgroups in the Han Chinese population. However, the BDNF Val66Met polymorphism, or another linked genetic locus, may reduce the severity of the psychopathology, in particular the negative symptoms of schizophrenia.
Acknowledgments
Dr. Hsin-An Chang and Dr. San-Yuan Huang contributed equally to this work as first authors.
This study was supported in part by the grants from National Science Council NSC 94-2314-016-016 (Dr. Huang), from Department of Health DOH94-TD-D-113-040 (Dr. Huang), DOH96-TD-D-113-031 (Dr. Huang), and from Tri-Service General Hospital TSGH-C94-76 (Dr. Huang), TSGH-C97-88 (Dr. Huang).