Multiple sclerosis (MS) is an autoimmune disorder of the brain and spinal cord that predominantly affects white matter and demonstrates a variable clinical presentation. Ever since its first clinical description, by Charcot and his school, there have been reports of emotional disturbances manifested in patients with MS, with depression being the most common phenomenon,
1,2 although this specific emotional disturbance is quite common in the general population, as well. There are still many questions regarding the specific biological connection between MS and depression, as well as the association between depression and MS exacerbations.
3,4MS represents a T-cell–mediated, autoimmune, demyelinating disease of the CNS. Interleukin-6 (IL-6) is a cytokine that has several effects on the neuroimmune system. T-cell activation seems to be linked to increased IL-6 binding. The upregulated IL-6 system is involved in antibody-mediated demyelinating pathways, because IL-6 is well known to enhance humoral immune response.
5 IL-6 is predominantly located at the sites of ongoing demyelination and immune activation. Although IL-6 exhibits several proinflammatory activities, indirect evidence suggests that the cytokine may also play an immunomodulatory role in inflammatory demyelinating disorders.
6 The key role of IL-6 in the pathogenesis of MS has also been demonstrated in experimental models, since the blockade of IL-6, using monoclonal antibodies against its receptors, inhibits the disease process.
7 A number of researchers have shown that the study of cytokine receptors is a much more reliable source of information regarding the immunological mechanisms, because of the short half-life of cytokines in body fluids.
8 Recent studies refer to higher levels of serum sIL-6R in patients of the relapsing–remitting group than in patients with non-inflammatory neurological diseases.
9Evidence indicates that an immune response, with an increased production of pro-inflammatory cytokines, often accompanies depression. Recently, researchers have demonstrated elevated IL-6 plasma concentrations during the acute state of depression, with decreases after remission.
10 An early theory proposed that pro-inflammatory cytokines secreted by activated macrophages, such as IL-6, can cause depression, forming the basis of the macrophage theory of depression.
11 IL-6 has also been used as a biomarker associated with the effectiveness of treatment in depression, demonstrating some changes during treatment with selective serotonin reuptake inhibitors.
12 Available evidence is consistent with three possible pathways: 1) depression-to-inflammation; 2) inflammation-to-depression; and 3) bidirectional relationships.
13IL-6 is one of the most important mediators of the acute-phase response. It is capable of crossing the blood–brain barrier and initiating synthesis of prostaglandin E2 in the hypothalamus. It is secreted by T-cells and macrophages to stimulate immune response to tissue damage leading to inflammation.
14 IL-6 signals through a cell-surface type I cytokine receptor complex as well as a soluble form (sIL-6R). Many neuronal cells are unresponsive to stimulation by IL-6 alone, but differentiation and survival of neuronal cells can be mediated through the action of sIL-6r. The sIL-6r/IL-6 complex can stimulate outgrowth of neurites and promote survival of neurons; hence, may be important in nerve regeneration through remyelination.
15Although there are a number of cytokines, such as Tumor Necrosis Factor (TNFα), interferon-α, IL-2, IL-6, and IL-10, that sometimes aggravate the symptoms of MS, there are others that may alleviate them.
16,17 IL-6, as one of the most widely investigated pro-inflammatory cytokine, was used by a great number of researchers as a reliable biomarker in both MS and depression literature.
5–7,10,12Many researchers indicate a possible correlation between IL-6 and MS, as well as between IL-6 and depression, but the possible correlation among the acute phase of MS, IL-6, and depression is not well investigated, maybe because depression is underdiagnosed, particularly during this phase of MS. The investigation of IL-6 and its soluble receptor may broaden the basis of understanding the underlying mechanisms of depression and the different phases of MS, and may therefore contribute to its treatment.
The aims of the present study were to investigate the levels of serum IL-6 and sIL-6R during the relapsing (acute phase) and the remitting phase of MS, as compared with those of healthy individuals, the presence of depression among the subjects in the three groups (relapsing MS patients, remitting MS patients, healthy individuals), the correlation between the serum levels of IL-6 and sIL-6R with the phase of the disease as well as with the presence of depression, and the correlation between the disability (as indicated by the Expanded Disability Status Scale [EDSS; Kurtzke]
18), cytokine levels, and depression.
METHODS
We examined 28 patients (14 men, 14 women), ranging in age from 22 to 50 years old, all suffering from definite MS, during an acute phase of the disease, according to the criteria of W.I. McDonald et al.,
19 demonstrating mild-to-moderate disability (mean EDSS value: 3.5), as well as 20 healthy individuals (control subjects), matched closely in age and education to our subjects and 14 patients currently in remission (
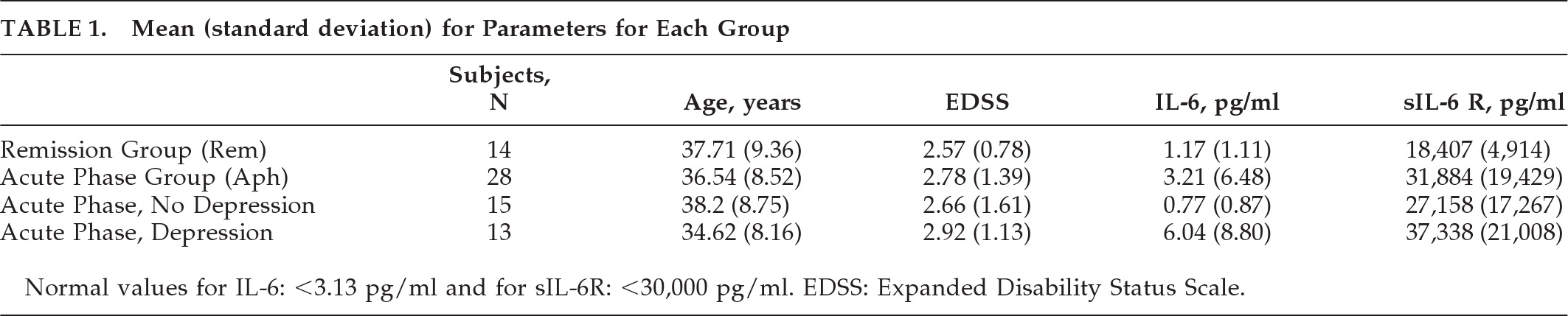
Table 1). All the examined subjects were white, Greek individuals. Since we tried to evaluate the presence of depression and cytokine values during the acute phase of the disease, the patients demonstrated mainly relapsing/remitting and secondary-progressive type of the disease. Also, we examined 14 MS patients (5 men, 9 women) ranging from 20 to 55 years old, in remission, demonstrating mild-to-moderate disability (mean EDSS: 3.0), mainly suffering from the relapsing/remitting and secondary-progressive type of the disease.
The study subjects gave informed consent for their participation; their anonymity was preserved. Patients were excluded if the structured interview revealed history of other neurological disease, drug or alcohol dependence, psychiatric disorder, or corticosteroid treatment within the time of examination, as well as severe motor, visual, or cognitive impairment that might substantially interfere with questionnaire completion. of the patients reported psychiatric or antidepressant treatment.
The diagnosis of depression was established and quantified by use of the Beck Depression Inventory (BDI) and the General Health Questionnaire-28 (GHQ-28), validated for Greek populations. Both are short, easy to complete, self-administered questionnaires in which the patients base their responses on their health status over the past 2 weeks, and not just during the last few days, in order to exclude transient symptoms. The patients were asked to fill out the form alone, in the morning, before the blood samples were drawn.
The BDI was designed as a screening device, rather than a diagnostic tool, but it is also used by healthcare providers to reach a quick diagnosis. Recent research has demonstrated that the psychometric properties of the Greek version of the BDI confirm it as a valid and reliable measure. According to the existing literature, the Greek version of the BDI had an overall Cronbach α of 0.906. The test–retest reliability, in terms of Spearman ρ, Pearson
r coefficient, and Kendall's tau-B was also satisfactory (p<0.0005). Correlations between the BDI and the Hospital Anxiety and Depression Scale (HADS) was 0.544 for the Anxiety subscale and 0.657 for the Depression subscale.
20,21 The use of the BDI may help assessment of depression in general hospital patients.
22 The questionnaire consists of 21 items that measure the severity of depression in adults; it is scored from 0 to 63. After careful research of the literature referring to depression in MS, we finally decided to use BDI scores >15 as the cutoff level for the diagnosis of depression. According to the Goldman consensus statement on depression in MS, this cutoff level is satisfactory in terms of sensitivity and specificity, and it was therefore appropriate in the context of the present study.
23,24The GHQ-28 represents a valid and practical screening instrument, developed to detect those likely to have or be at risk of developing psychiatric disorders. It assesses somatic symptoms, anxiety, insomnia, social dysfunction, and severe depression.
25,26 Each positive response counts as 1 point, and the maximum score for each of the four groups is 7. Total scores range from 0 to 28. Higher scores indicate a greater probability of psychiatric distress. Total scores that exceed 4 out of 28 suggest probable distress.
27,28 The GHQ 28-item version, which was used in this study, has been validated, demonstrating good psychometric properties within Greek populations. The internal consistency, item-by-item and the subject-by-subject analysis in 100 consecutive patients, have shown that the English and the Greek version are equivalent. The best cutoff points for the Greek version of the GHQ-28 has been reported as 4/5.
29The patients who were diagnosed as depressed demonstrated both BSI scores >15 and GHQ-28 scores >4, as indicated by previous investigations in Greek populations.
22,25,29On the same day that depression was evaluated, before 10:00 A.M., blood samples were drawn to determine the levels of IL-6 and sIL-6R in the serum. Venous blood was centrifuged for 10 minutes at 2,000 g, and the sera samples were then stored in aliquots at –40°C until their examination. The evaluation of the IL-6 and its receptor was carried out by the use of commercially available immunodetective ELISA kits (QuantiGlo, hIL6 and Quantikine hIL6 sR Immunoassay kits; R&D Systems, U.S.A.). Both the determination of depression and the blood samples were obtained before the patients received corticosteroids and without demonstrating obvious inflammation (normal values of leukocytes and C-reactive protein;
Table 1).
Statistical Evaluation
The parameters under evaluation were EDSS, IL-6, sIL-6R, and depression. There were two main groups: MS patients in remission (Rem) consisted of 14 subjects, and MS patients in relapse (acute phase: APh) consisted of 28 subjects. We divided the APh into two subgroups on the basis of the presence of depression. The first subgroup consisted of 13 individuals with depression, and the second consisted of 15 subjects without depression.
The collected data were compared according to the following pairings: 1) Remission Group (Rem) versus Acute-Phase Group (APh); 2) Remission Group versus Acute Phase/No-Depression (APh No Depr.); 3) Remission Group versus Acute Phase/Depressed (APh Depr.); and 4) Acute Phase/No-Depression versus Acute Phase/Depressed.
The distribution of the collected data were assessed with the Kolmogorov-Smirnov Z and Shapiro-Wilks test of the SPSS 16.0. An independent-samples t-test (two-tailed, with a 95% confidence interval) was used for the statistical evaluation of EDSS and sIL-6R. For the evaluation of IL-6, we used the Mann-Whitney test. Pearson correlation was used to establish the relation between EDSS and IL-6R, and Spearman's correlation coefficient was used to evaluate the relationship between EDSS and sIL-6R.
RESULTS
The present study concerned the determination of IL-6 and its soluble receptors in the sera of 28 MS patients during the acute phase of the disease, before receiving corticosteroids: 14 MS patients in remission and 20 healthy-control participants.
We also examined the presence of depression in all three groups. Both MS groups demonstrated mild-to-moderate disability according to the EDSS; 33.5% of the MS patients (46% of the APh and 21% of the Rem) and 10% of the healthy individuals were diagnosed as depressed.
The cytokine levels were found to be normal for all the healthy control subjects, although 10% of them demonstrated depression. EDSS levels were similar in all groups, and no relationship between EDSS and cytokine levels or presence of depression was demonstrated.
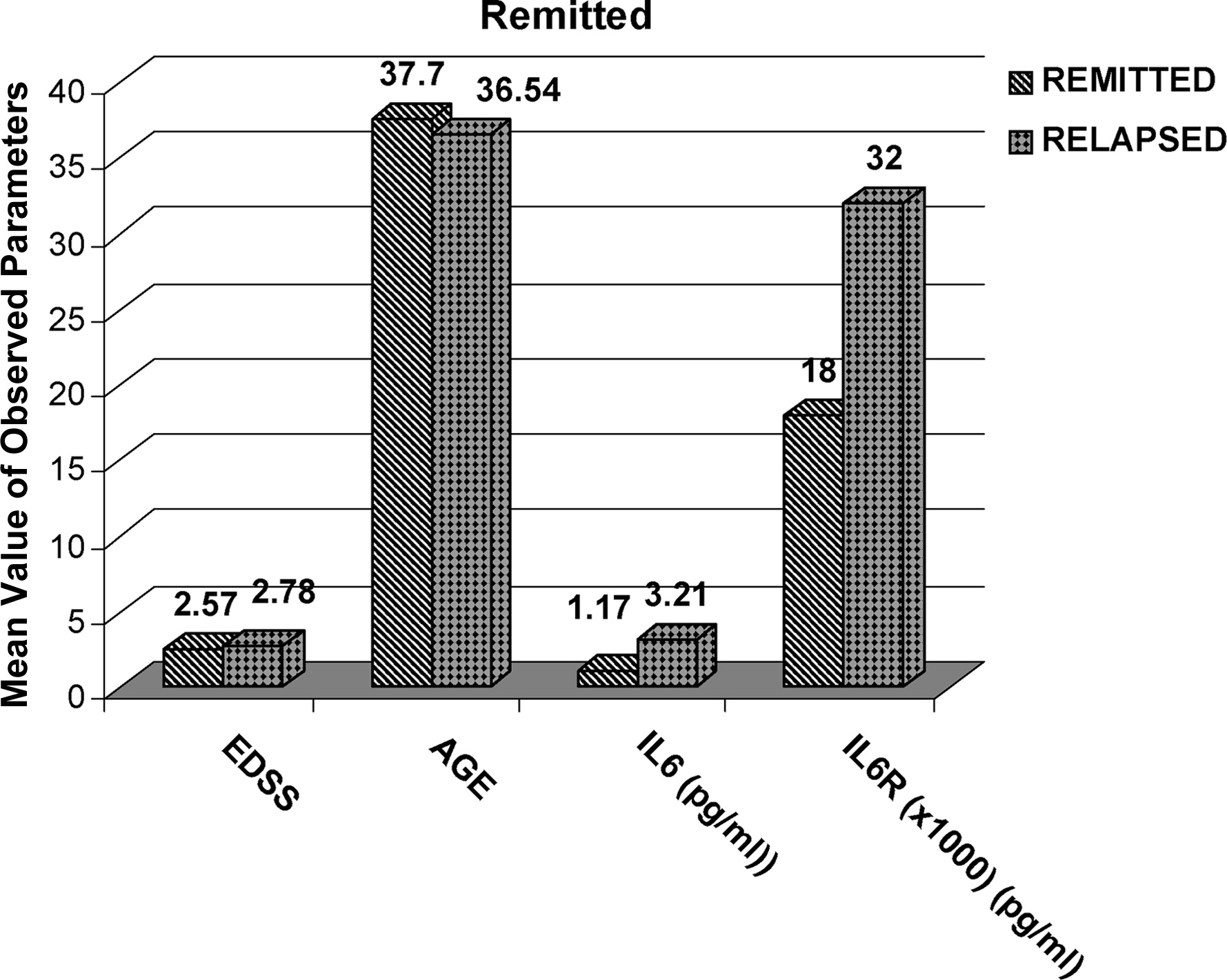
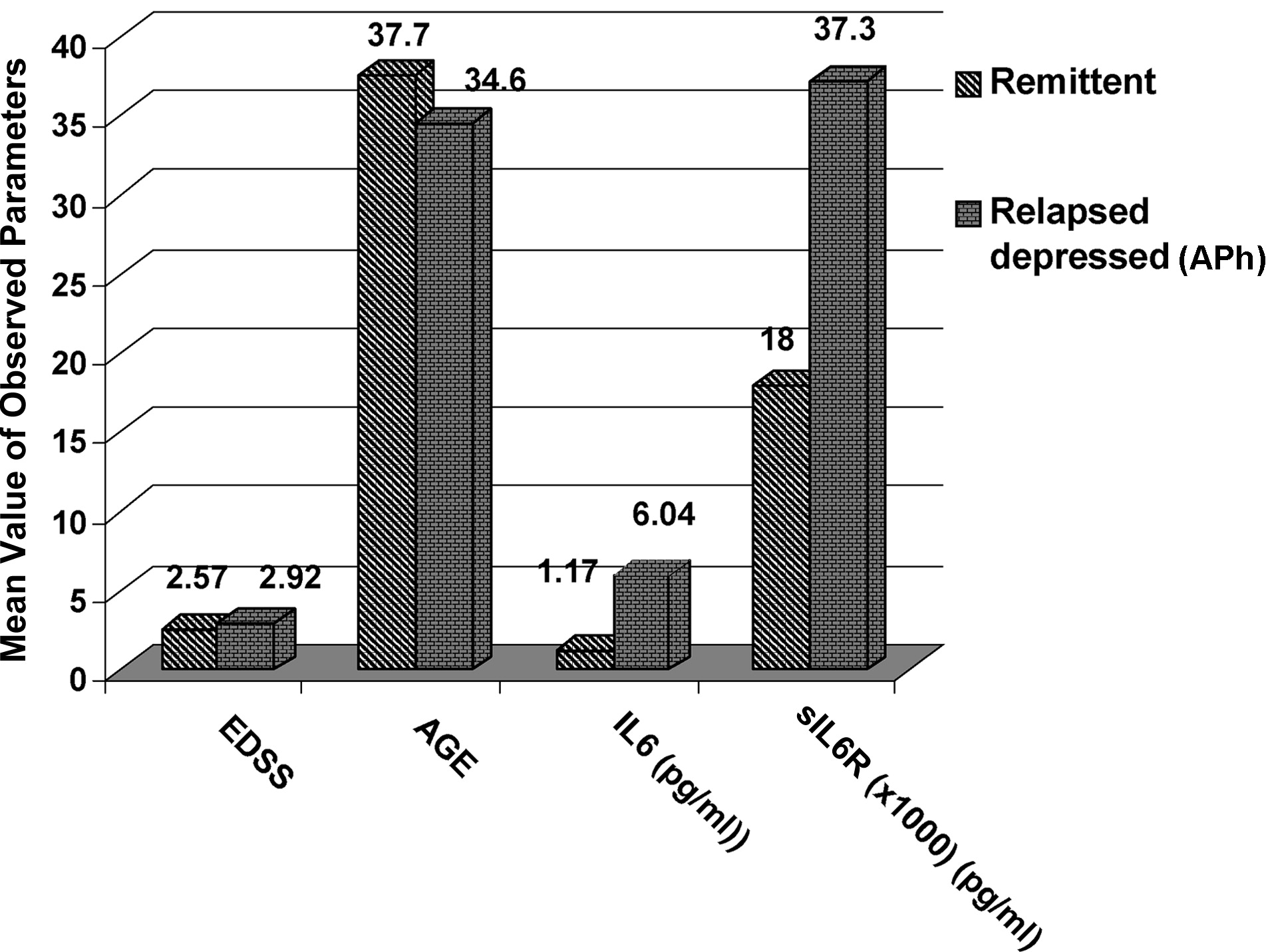
The sIL-6R levels were higher in the APh than in the Rem group (
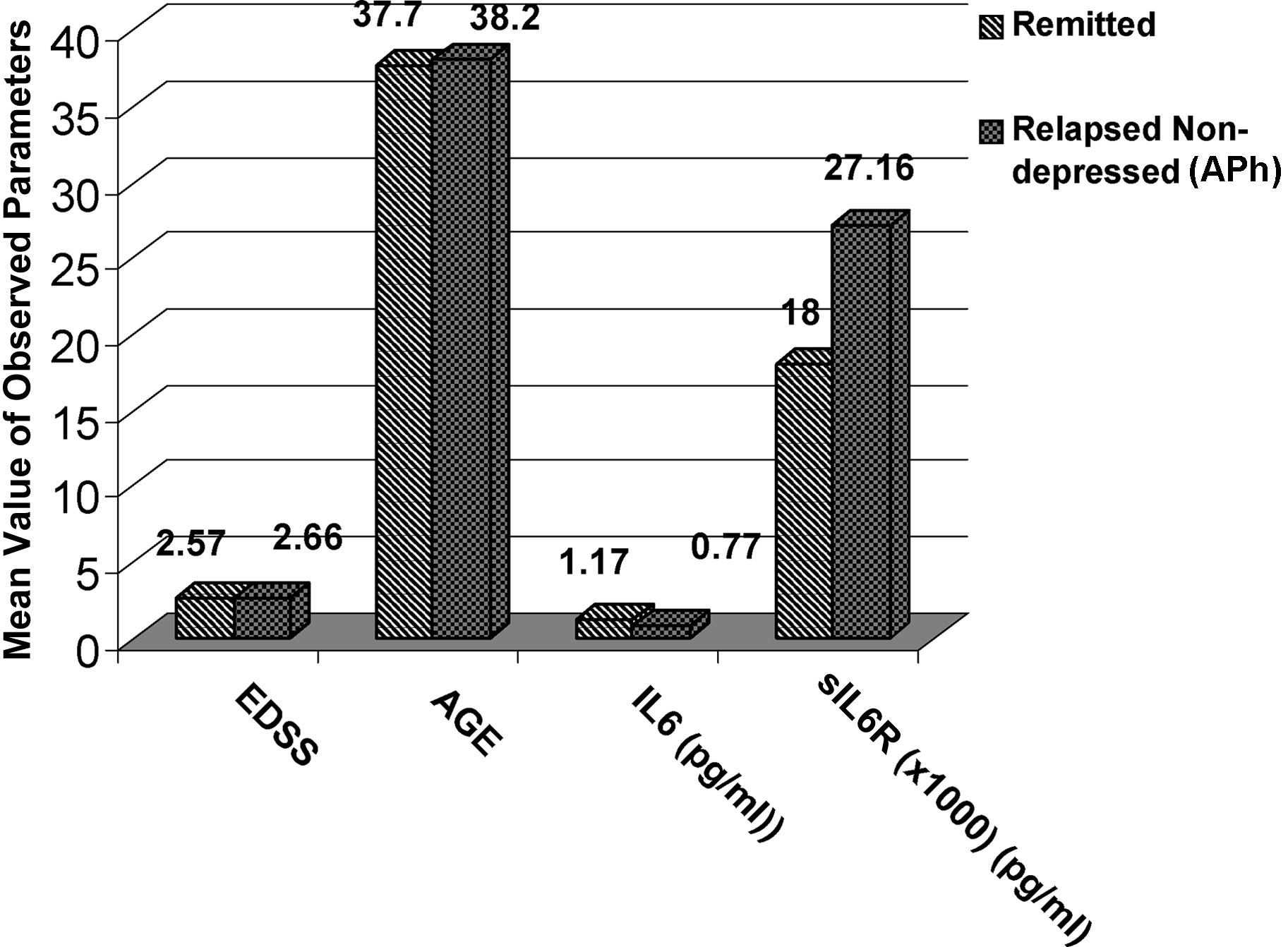
Figure 2). This difference is more accentuated if we compare the Rem with the APh Depr group (
Figure 3).
•.
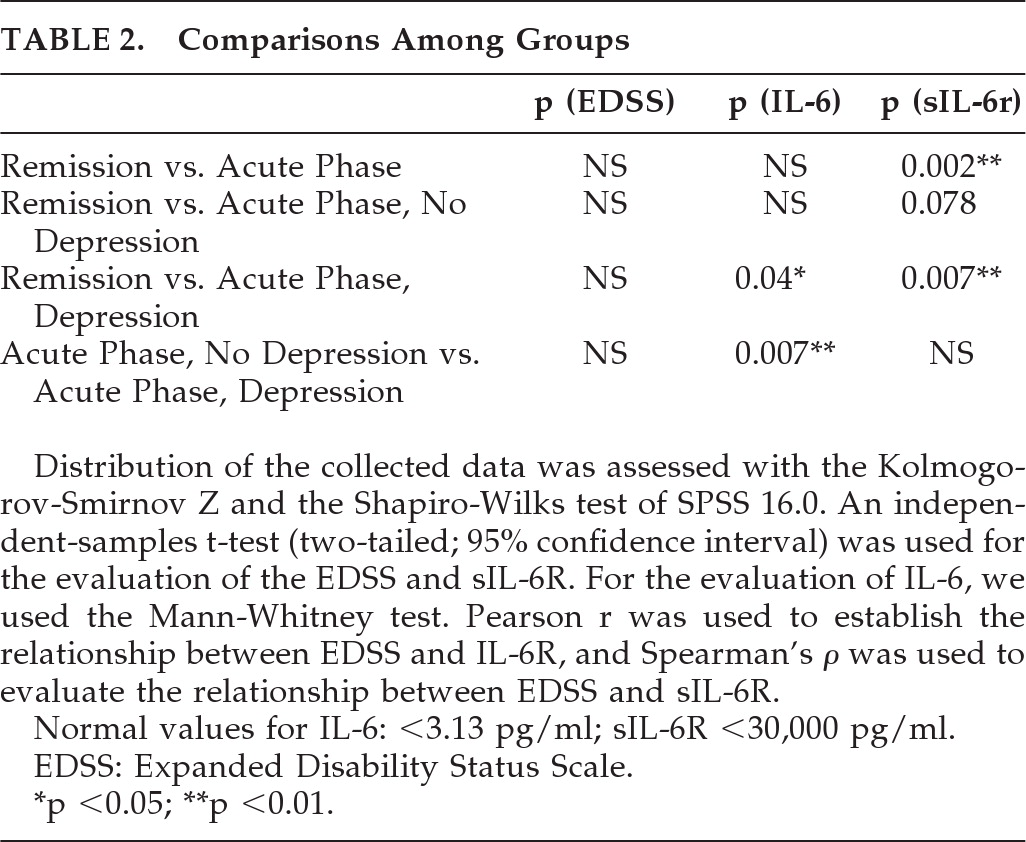
The first comparison was between the Remission Group and Acute Phase Group, and it revealed no difference on EDSS, increased values, and no statistically significant difference for IL-6, whereas there was a statistically significant difference for sIL-6R (p=0.002).
•.
The second comparison was between the Remission Group and the Acute Phase-No Depressed Group, and it revealed no statistically significant difference for EDSS, IL-6, or sIL-6R, although the values of sIL-6R in the APh no Depr were higher (p=0.07), but not statistically significantly different from those demonstrated in the Rem.
•.
The third comparison was between the Remission group and the Acute Phase-Depressed Group, and it revealed statistically differences for both IL-6 (p=0.04) and sIL-6R (p=0.007).
•.
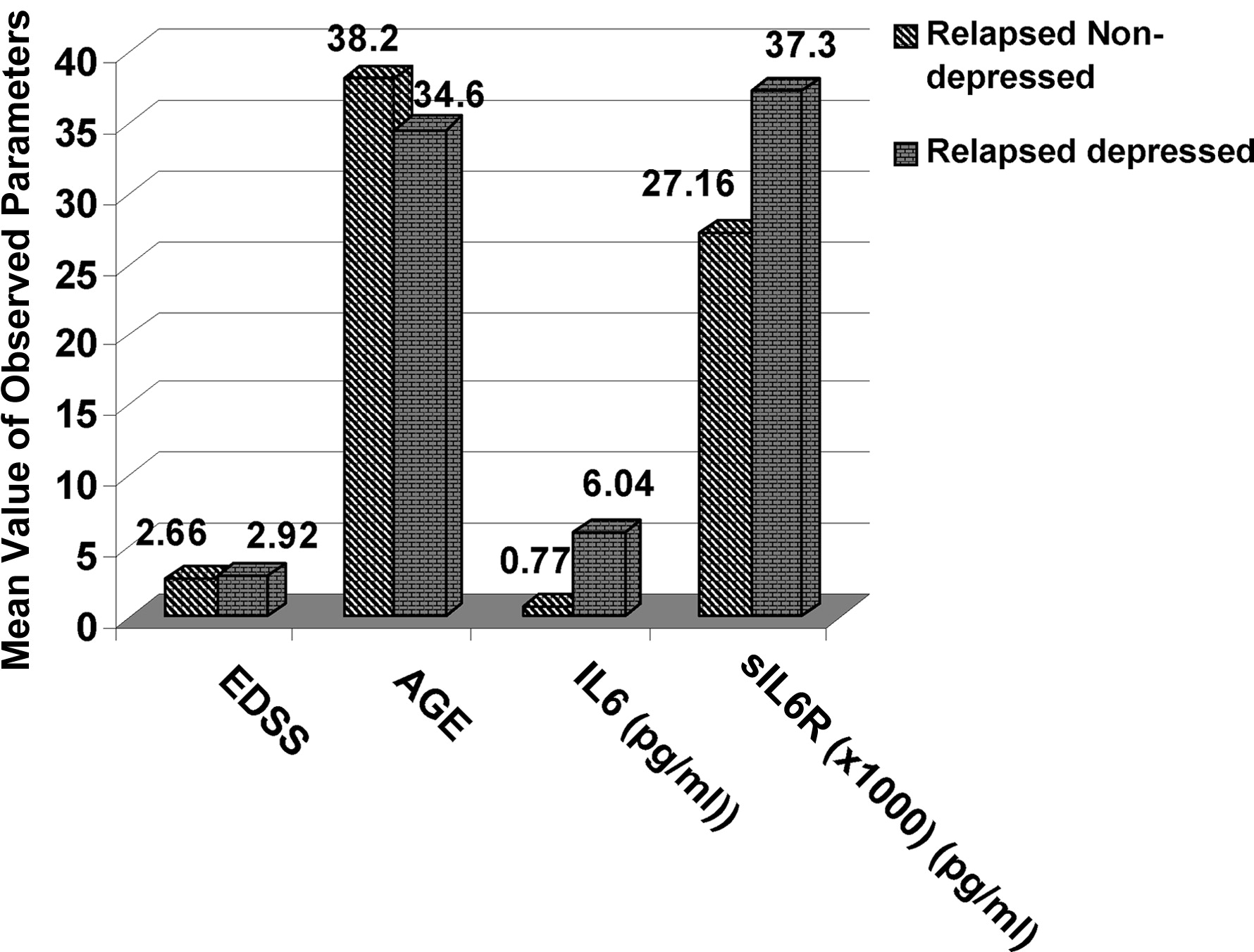
The fourth comparison (
Figure 4) was between the Acute Phase-Depressed and the Acute Phase No-Depression group, and it revealed a statistically significant difference for IL-6 values (p=0.007) and increased values, but not statistically significant different, for sIL-6R in the Acute Phase, Depressed group (p=0.17).
DISCUSSION
Recent reports have demonstrated that IL-6 acts as an endogenous pyrogen during the acute stages. It has been linked with the differentiation of the immune response; it can be detected within the perivascular inflammation cells in the acute demyelinated loci characterized by inflammation and demyelination, and it represents an important marker for the acute phase of the disease. Its action is regulated by the expression of its receptor, both on the cell surface and in its soluble form, which, unlike the soluble receptors for other cytokines, tends to stabilize and strengthen the effect of IL-6.
1,14,17The results of the present study, demonstrating elevated values of sIL-6R in the acute phase group, (APh) as compared with the group in remission (Rem; p=0.002), may suggest that IL-6, and particularly its soluble receptor, may be reliable diagnostic marker for the acute phase of MS. Also, our study indicates that depression is much more common in MS patients than in healthy-control participants, particularly during the acute stage of the disease. Since there was no statistically significant difference between the Rem group and the non-depressed APh group, whereas there was significant difference in both IL-6 (p=0.04) and sIL-6R (p=0.007) when we compared the Rem group and the APh depressed group, we could confirm a possible connection between the acute phase of MS and elevated levels of the cytokines.
Their relation to depression is evident, but the pathogenetic base of this connection is more complicated. There may be a direct connection between elevated levels of cytokines and depression, but we should also consider the emotional reaction of the patients to the burden of the disease. The fact that IL-6 and sIL-6R levels were not increased in the depressed control subjects may indirectly support the idea of emotional reaction as the cause of depression. This finding is in contrast with reports that depression is related to elevated levels of cytokines in normal individuals.
2,4 The discordance is probably due to the different inclusion criteria for depression and the small sample size. On the other hand, the significant difference found in IL-6 (p<0.01) between Rel Depr and Rel No-Dep MS patients supports a relationship between elevated levels of the cytokines and depression. The positive association between depression and inflammation may also be the result of a complex, bidirectional process in which CNS correlates with depression, which alters immunity, and vice versa. Much evidence supports this reciprocal hypothesis.
13 Our results also demonstrated no correlation between disability status, which represents mainly axonal loss (degenerative stage), and the IL-6 and sIL-6R levels connected mainly with the inflammatory stage of the disease.
One limitation of the present study is represented by the diagnostic criteria used for depression. We used self-administered questionnaires without further clinical evaluation, as other researchers have done.
30 Also, the sample size, although it was sufficient as compared with previous studies, could be greater in order to demonstrate more statistical significant differences among the examined groups. We examined only IL-6 and sIL-6R as representatives of the acute stage of MS, although there is a complicated cytokine network that contributes to the pathogenesis of both MS and depression.
CONCLUSION
Given the overlap of symptoms between depression and MS, the diagnosis of depression in patients with MS is often complicated and underestimated. Because depression is common and can temporarily fluctuate in patients with MS, healthcare providers must have the necessary tools to make timely and accurate diagnoses.
Our results indicate that the diagnosis of depression in MS patients, particularly during the acute phase of the disease, is critical before initiation of anti-inflammatory or immune-therapy. The treatment of depression may provide a novel disease-modifying strategy, and therapy with corticosteroids may affect the long-term prognosis of both depression and MS.
Undoubtedly, more research is required, in order to investigate the association between MS, inflammation, and depression, and even more cytokines may intervene in the spectrum of depressive phenomena in MS or may be beneficial in the therapeutic approach to the disease.