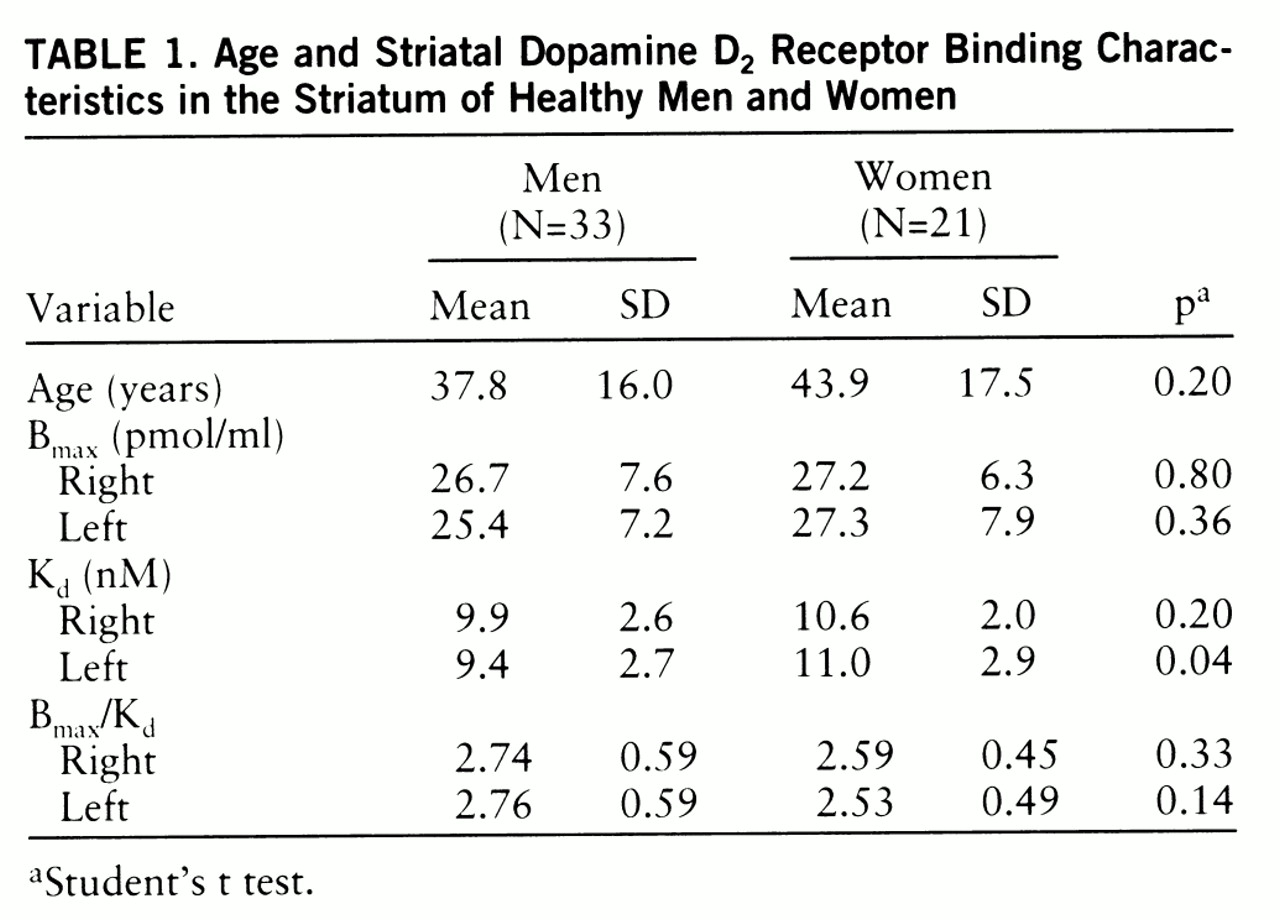
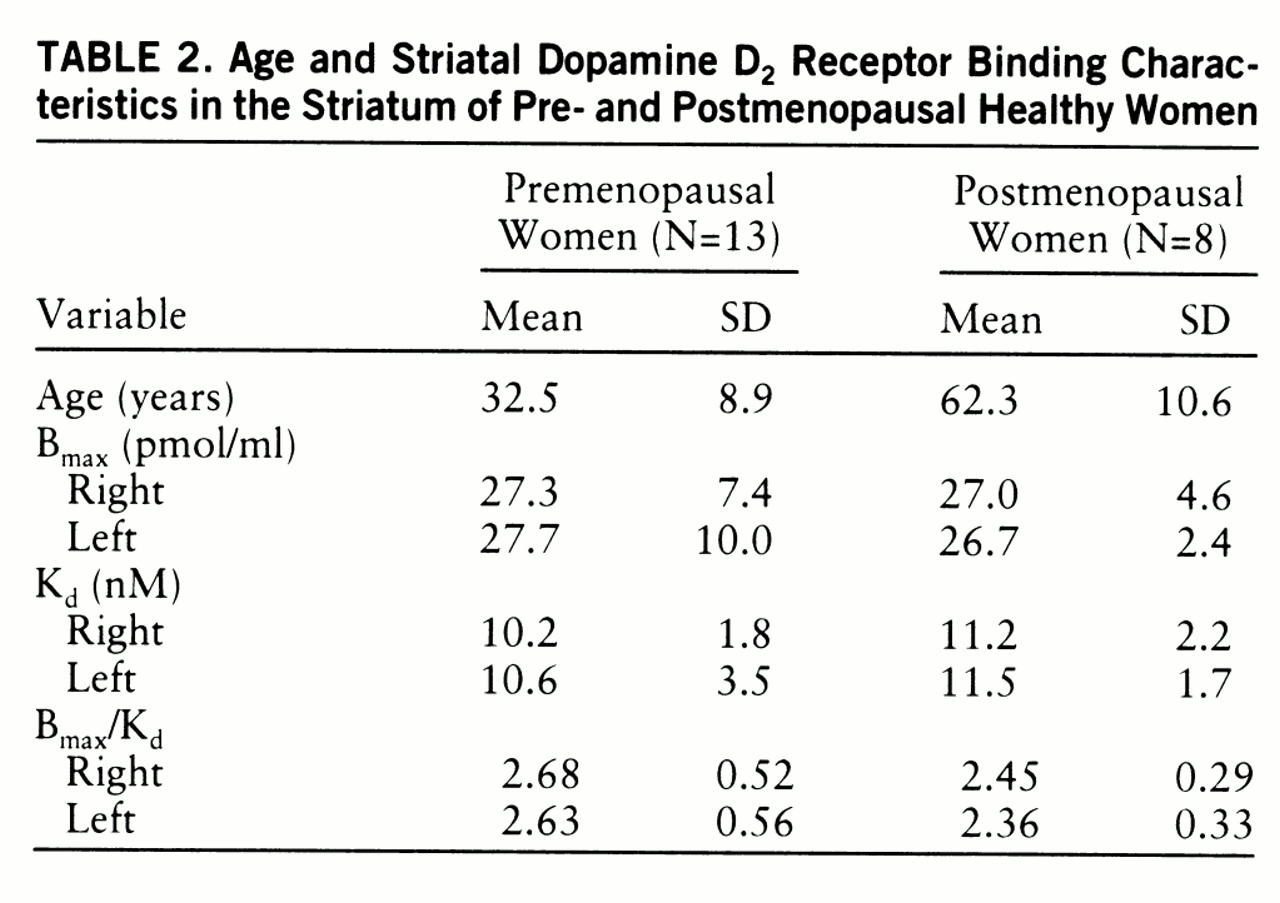
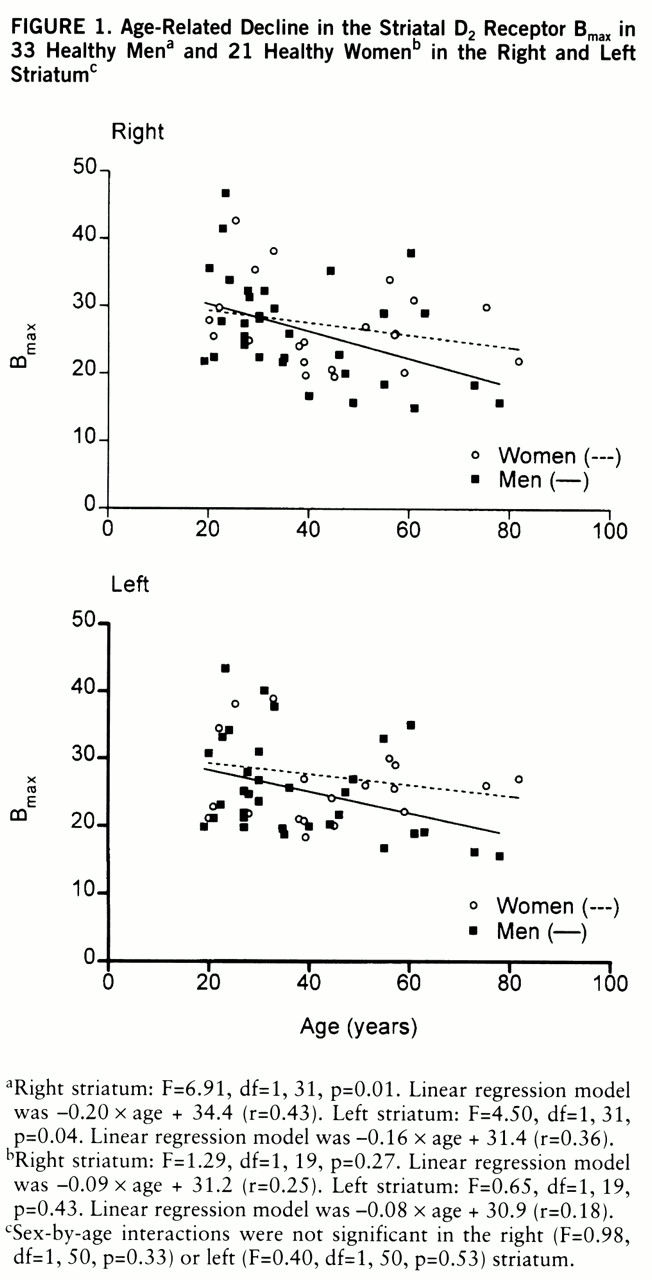
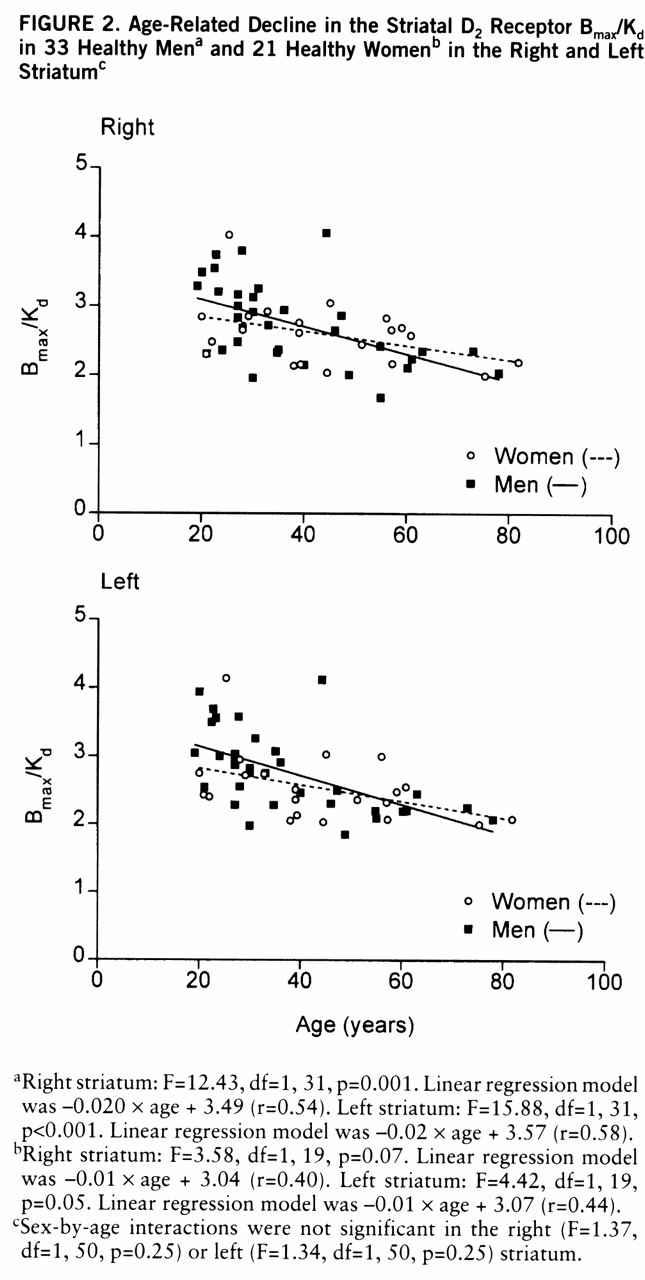
Age and D2 Receptor Binding Characteristics
The results of this study confirm the significant decline in D
2 receptor B
max and B
max/K
d values as a function of age in human striatum. The effect of aging on the decline in striatal D
2 receptor density, but not affinity, has been firmly documented in humans in postmortem studies of brains (
25–
27) and in vivo studies through use of PET (
19,
20,
28–
31). Similar age-related decreases in the activity of tyrosine hydroxylase (
32), levels of dopamine concentration (
15,
32,
33), and dopamine uptake (
34,
35) have been described, whereas changes in dopa de~carboxylase activity have been inconsistent. Some studies have suggested that dopa decarboxylase activity remains unaltered with age (
36–
38), and others have suggested that it decreases slightly (
39).
It has been suggested that the age-related decline in D
2 receptor binding in vivo is different in men and women (
19,
20). In a recent in vivo study, covering a relatively narrow age range (20–38 years), neither the D
2 receptor characteristics nor the influence of age was found to be different in men and women (
21). In the present study the large age range of subjects, from 19 to 82 years, revealed an age-related decline in D
2 receptor density and binding potential but not in affinity in vivo. B
max and B
max/K
d tended to decline with age twice as fast in men as in women on average, and a significant age-related decline was found only among men. However, the gender difference in age-related decline in density and binding potential did not reach statistical significance.
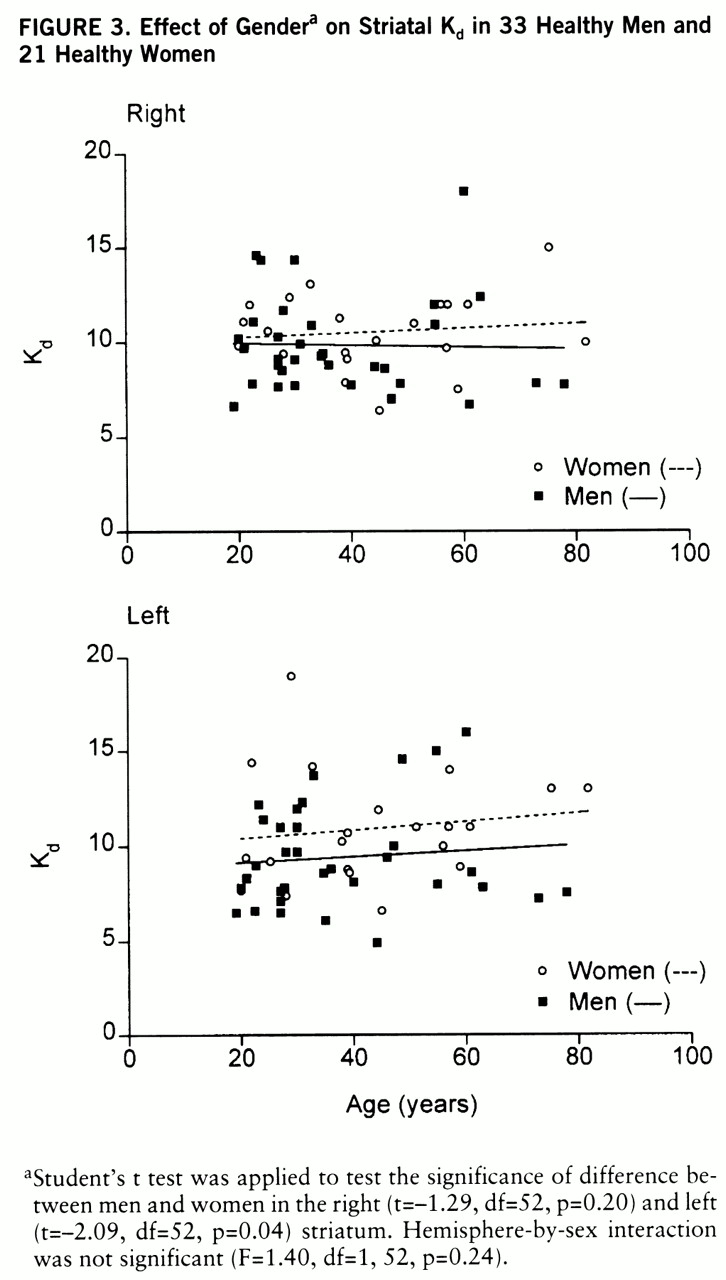
Gender and D2 Receptor Binding Characteristics
Striatal [
11C]raclopride binding has been shown to be sensitive to endogenous dopamine fluctuations in several studies in vivo (
40–
42). This property has been used to assess changes in endogenous dopamine concentration after pharmacological interventions in human brain in vivo (
43,
44). In the present study, the lower affinity of [
11C]raclopride in women than in men suggests a gender difference in basal endogenous dopamine concentration in the left striatum. This conclusion is based on a reversible ligand-receptor interaction with competition by endogenous dopamine. This results theoretically in elevated K
d and unchanged B
max values. It has also been suggested that dopamine noncompetitively inhibits [
3H]raclopride binding to D
2 receptors in vitro, thus decreasing B
max, while K
d remains unchanged (
45). Ross and Jackson (
46) observed in their ex vivo study in mice that the reduction of synaptic dopamine concentration with reserpine and γ-butyrolactone reduced K
d values of [
3H]raclopride to D
2 receptors because of the competition between dopamine and raclopride. However, the 50% increase in B
max values after dopamine depletion by reserpine suggested that a pool of D
2 receptor sites was not available for raclopride to bind when occupied by dopamine. Recent PET studies in monkeys measured with [
11C]raclopride and the equilibrium method showed that the reduction of dopamine concentration in brain by reserpine resulted in higher affinity but no change in D
2 receptor B
max (
47). This corresponds to competitive inhibition between [
11C]raclopride and dopamine and supports the interpretation of elevated striatal dopamine concentration in women.
Protein structure may affect the affinity of the ligand for the receptor. Our subjects have been studied for structural mutations in the coding region of the D
2 receptor gene (
48). The only subject, a 20-year-old man, possessing protein sequence variation in Ser
311→Cys showed normal affinity as well as age-corrected B
max and B
max/K
d characteristics in vivo compared to the mean values of all subjects, indicating that structural variation in the coding region did not cause the gender difference in the present K
d values.
The sex-related change in endogenous dopamine concentration may be due to hormonal effects on dopaminergic functioning in the striatum. Gonadal steroid hormones are known to modulate dopaminergic transmission in the rat. Biosynthesis (
49), concentration (
50,
51), degradation (
52), uptake (
53,
54), and receptor density (
55) of dopamine have been shown to fluctuate during the estrous cycle in the rat. Thus far, such differences in humans have not been confirmed. In a PET study, the binding rate constant (k
3) of [
11C]N-methylspiperone to D
2 receptors was reported to fluctuate during the menstrual cycle in women (
20). In the current study detailed data on menstrual cycling were not available for all women; however, the effect of menopausal status was analyzed by separately comparing the binding parameters of premenopausal and postmenopausal women to those of all men. The variability in B
max and B
max/K
d values was, in general, larger among premenopausal than postmenopausal women, probably because of fluctuation in gonadal steroid hormones during the menstrual cycle; however, this remains to be proven.
The role of gender has become an extensively studied variable in schizophrenia research in the last decade. Numerous studies have noted differences in the age at onset, treatment response, course of illness, and outcome between men and women (
11,
56,
57). The present results may bear relevance to a gender difference in schizophrenia. The prognosis of schizophrenic women is regarded as better than that of men, and female patients maintain a higher level of social functioning. Similarly, it has been indicated that men are more likely than women to be affected by negative symptoms of schizophrenia. Because positive (psychotic) symptoms of schizophrenia may derive from overactivity of the dopaminergic system, the current antidopaminergic antipsychotic drug treatment may be better targeted for women and therefore improve the prognosis.
Schizophrenic symptoms appear to correlate with low levels of estrogen across menstrual cycle (
58,
59). These observations suggest that estrogen protects against the development of schizophrenia in female subjects (
11,
60,
61). Biochemical and behavioral studies in animals have demonstrated that estrogen modulates dopaminergic transmission in striatum; however, both activating and inhibiting effects have been documented. In women, the main effect of estrogen is thought to be antidopaminergic, but the mechanism underlying this interaction is poorly understood. Our data on the lower affinity of [
11C]raclopride in women than in men suggest a gender-related difference in synaptic dopamine concentration in the left striatum. The increased dopamine concentration in vivo, especially in postmenopausal women, supports the view that loss of estrogen has a facilitative effect on dopaminergic transmission. According to the dopamine hypothesis of schizophrenia/psychosis, this may have implications for increased vulnerability for psychotic disorders in elderly women, since women are at higher risk than men, e.g., to develop late-onset schizophrenia (
62,
63).
The difference in the dopamine D
2 system may also affect vulnerability to alcoholism. Alcoholic men have been reported to have a low striatal D
2 receptor density (
24,
64). It can be speculated that higher dopamine tone in women may play a role in lower vulnerability to alcohol dependence in women. Our results are consistent with the fact that the male to female ratio for alcohol-related disorders is about 2 to 1 or even 3 to 1.
Finally, the gender difference in D2 receptor affinity should be regarded as a preliminary finding, since the difference did not remain significant when a Bonferroni correction for multiple comparisons was applied. Further studies, including also detailed endocrinological data, are required to delineate the basis of variation in endogenous dopamine concentration between men and women. This may shed light on biological liability factors in psychiatric disorders like schizophrenia and alcohol and substance dependence.