Previous twin and adoption studies have found moderate to strong genetic influences on alcoholism among men, with heritability estimates of 40%–60%. These include studies using archival or registry data
(1–
5), clinically ascertained samples
(6–
11), and a sample of volunteer Australian twins
(12). Two studies that did not support strong genetic influences had limitations of small sample size
(13) and incomplete ascertainment of cases
(14). Several studies
(2,
4,
8,
10,
11) found evidence for shared environmental effects in addition to genetic effects, particularly when alcoholism was defined broadly.
Studies based on archival data or treatment samples are likely to be weighted toward more severe forms of alcoholism, and cases may be selected for excess comorbidity with other disorders
(15). These cases account for only a small proportion of the morbidity and social costs associated with misuse of alcohol
(16). We addressed this issue by studying, for the first time in a population-based U.S. sample, the etiology of alcohol-related disorders in personally interviewed male twins.
METHOD
This report is based on data collected as part of a new study of adult twins from the Virginia Twin Registry. The registry was formed by means of a systematic search of all Virginia birth certificates since 1918. Subjects from multiple births are matched by name and birth date to state records to obtain addresses and telephone numbers. Individuals were eligible for participation in this study if one or both of a pair were successfully matched, if they were a member of a multiple birth that included at least one male, if they were Caucasian, and if they were born between 1940 and 1974. Of 9,420 eligible individuals, 6,856 (72.8%) were interviewed, 1,163 (12.3%) refused to participate, 385 (4.1%) did not agree to participate within the study time limit, 863 (9.2%) could not be located, and 153 (1.6%) were deceased or too ill to be interviewed. Thus, of 8,404 twins contacted and available for participation, 81.6% were successfully interviewed.
This report is based on 3,516 male twins, triplets, and quadruplets from male-male pairs with complete data on alcohol diagnosis. The sample included 3,012 subjects from 1,514 complete pairs (861 identical and 653 fraternal) and 504 subjects whose co-twins were not interviewed. (The number of pairs includes 12 created by combining all possible pairs from four all-male triplet sets.) Excluded from the analyses were 27 males with incomplete alcohol data and 3,313 male and female twins from opposite-sex pairs.
At the time of interview (1993–1996), the subjects were 18–56 years old (mean age=35.1 years, SD=9.2) and had a mean of 13.4 years (SD=2.6) of education. Fifty-nine percent were married, 13% separated/divorced, 28% single, and 0.5% widowed. Most subjects were interviewed by telephone, but about 5% were interviewed in person because of subject preference, residence in an institutional setting (usually jail), or not having telephone service.
This project was approved by our local institutional review board. Subjects were informed about the goals of the study and provided verbal consent before the telephone interviews and written informed consent before collection of DNA samples.
Measures
Lifetime alcohol abuse and alcohol dependence were assessed by structured interview. Diagnostic criteria were adapted from standard instruments
(17,
18) and structured to permit evaluation of both DSM-III-R-defined and DSM-IV-defined diagnoses. Subjects were assessed for alcohol-related diagnoses if they 1) reported that their maximum single-day intake was 13 or more drinks, and/or 2) answered yes to any of three screening questions: “Have you ever had a period in your life when you drank too much?…when you drank instead of spending time with hobbies, family, or friends?…when someone else objected to your drinking?” Diagnostic criteria were evaluated for the time “when you used alcohol the most” (number 1) or when “this problem was at its worst” (number 2).
Four measures of alcohol consumption were obtained for the year when the subject drank the most: frequency (number of days on which alcohol was consumed in a typical month), quantity (typical number of drinks per drinking occasion), maximum single-day quantity, and monthly consumption (calculated as a weighted sum of the frequency of consuming 1–3, 4–6, 7–9, 10–12, and 13 or more drinks).
Interviewers had a master’s degree in social work, psychology, or another mental health-related field or a bachelor’s degree in one of these areas plus 2 years of relevant clinical experience. They received 40 hours of classroom training plus regular individual and group review sessions. Two senior staff members reviewed each interview for completeness and consistency. Members of a twin pair were interviewed by different interviewers who were blind to clinical information about the co-twin.
Pairs were classified as identical (monozygotic) or fraternal (dizygotic) on the basis of a computer algorithm of questionnaire responses previously validated on a parallel sample of female-female twin pairs
(19). We validated the application of the algorithm to the male sample by analysis of 15 highly informative DNA polymorphisms in a random sample of 184 twin pairs. The algorithm correctly classified 177 (96.2%) of the pairs.
Statistical Analysis
We present information about twin resemblance in several ways. Proband concordance is the proportion of affected individuals among the co-twins of affected twins (probands). This statistic does not use the information available from the proportion of pairs in which both twins are unaffected. All of the information is summarized by an odds ratio—the risk of being affected among co-twins of affected twins compared to the risk of being affected among co-twins of unaffected twins.
We use a standard liability-threshold model to estimate the genetic and environmental contributions to twin resemblance. For a categorical characteristic such as diagnosis, the estimates are for the resemblance of twins in a pair for their liability to develop the disorder
(20). Liability is assumed to be continuous and normally distributed in the population, with individuals who exceed a theoretical threshold expressing the disorder.
Individual differences in liability are assumed to arise from three sources: additive genetic (A), from genes whose allelic effects combine additively; common environment (C), which includes all sources shared by members of a twin pair, including family environment, social class, and schools; and specific environment (E), which includes all remaining environmental factors not shared within a twin pair plus measurement error. Monozygotic twins within a pair resemble each other because they share all of their A and C components, while dizygotic pairs share all of their C and (on average) one-half of their A components. Comparing the resemblance of monozygotic and dizygotic twins provides estimates of each source’s contribution to the population variance in liability. It is possible to include nonadditive genetic effects (dominance, epistasis), but these have not been implicated in prior studies of alcohol abuse/dependence, and their inclusion did not improve the fit of our models, so we do not consider them further.
Our model assumes independence and additivity of the three components, absence of assortative mating, equality of shared environmental effects, and no age effects. Assortative mating is the tendency for people to choose mates nonrandomly. If spouses choose each other on the basis of characteristics that are genetically influenced, the genetic correlation for these characteristics among dizygotic twins will be higher than 0.50, and the genetic component will be underestimated. Spousal resemblance for alcohol dependence was modest (correlations of –0.21 to 0.27) among parents of female-female twins studied previously
(21), so a large impact of assortative mating seems unlikely.
The equal environment assumption requires that monozygotic and dizygotic pairs be equally similar in the etiologically relevant aspects of their shared environments. We test this assumption by studying whether, when zygosity is controlled, pairs with greater adult or childhood environmental similarity are more similar for alcoholism.
Prior studies have found differences between cohorts in risk of alcoholism, and the younger subjects in our sample have not completed the risk period. We address this by testing the invariance of the prevalences and biometric parameters across age groups.
Models were fitted directly to contingency table data with the use of weighted least squares in the program Mx
(22). Alternative models were evaluated by comparing the difference in their chi-square values relative to the difference in their degrees of freedom, according to the principle of parsimony—models with fewer parameters are preferable if they do not provide significantly worse fit. We operationalized parsimony by using the AIC statistic
(23), calculated as χ
2–2 df.
RESULTS
Diagnostic Prevalences
Lifetime prevalences were as follows: according to DSM-III-R criteria, alcohol abuse=7.6% and alcohol dependence=27.4%; according to DSM-IV criteria, alcohol abuse=12.0% and alcohol dependence=24.0%. To address the convergent validity of these classifications, we examined the association between diagnosis and alcohol consumption for the year of heaviest drinking. The 3,473 subjects who reported any lifetime alcohol consumption were divided into three groups: one group included 1,015 subjects who met the DSM-III-R or DSM-IV criteria for alcohol dependence; the second included 318 subjects with DSM-III-R or DSM-IV alcohol abuse (but not alcohol dependence); and the third included 2,140 nonabstainers without any diagnosis.
The three groups differed significantly in consumption frequency (days per month) (alcohol dependence group mean=22.3, SD=8.4; alcohol abuse group mean=18.4, SD=8.6; no-diagnosis group mean=9.6, SD=8.5; F=829, df=2, 3470, p<0.0001; R2=31.8%) and in typical quantity (number of drinks per drinking occasion) (alcohol dependence group mean=9.9, SD=7.3; alcohol abuse group mean=7.3, SD=4.6; no-diagnosis group mean=4.3, SD=4.0; F=410, df=2, 3468, p<0.0001; R2=18.8%). They also differed significantly in single-day maximum number of drinks (alcohol dependence group mean=20.7, SD=11.4; alcohol abuse group mean=16.5, SD=8.3; no-diagnosis group mean=9.3, SD=7.2; F=616, df=2, 3470, p<0.0001; R2=25.8%) and in total drinks per month (alcohol dependence group mean=252, SD=209; alcohol abuse group mean=160, SD=125; no-diagnosis group mean=57, SD=84; F=740, df=2, 3468, p<0.0001; R2=29.5%).
Potential Biases
Twins whose co-twins were not interviewed were older (mean age=35.8 years, SD=9.2, versus 34.6 years, SD=9.3; t=13.0, df=3516, p<0.0003; R2=0.4%) but did not differ significantly in the prevalence of any diagnosis, suggesting that restricting the analyses to complete pairs did not bias the results.
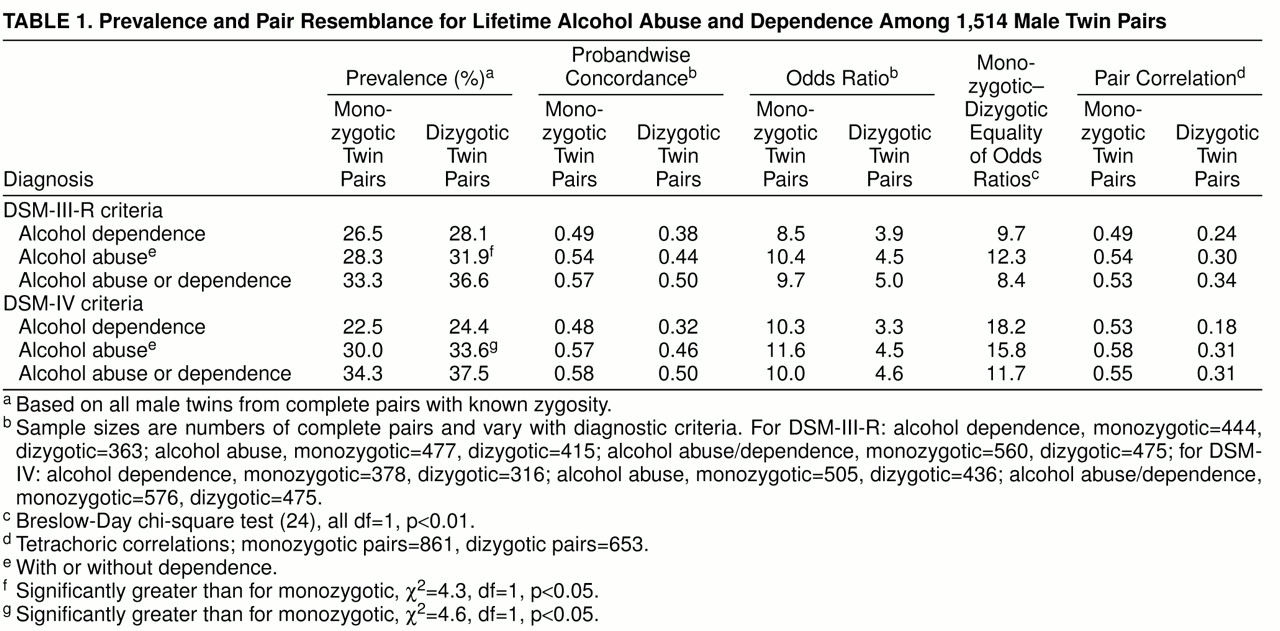
Dizygotic twins had slightly higher prevalences for each classification (
table 1), but these were significant only for alcohol abuse. Our models required equal thresholds across zygosity; any threshold differences contribute to model misfit.
We tested the validity of the equal environment assumption by studying the association between diagnosis and measures of environmental similarity during childhood (sharing a bedroom, sharing playmates, dressing alike, being in the same classroom) and adulthood (age at which the twins stopped cohabiting, frequency of past-year contact). Logistic regression models were used to predict pair concordance for diagnosis (DSM-III-R and DSM-IV alcohol abuse/dependence and alcohol dependence) from zygosity and the interaction of zygosity with each environmental measure. Of 24 tests conducted (four diagnoses multiplied by six predictors), we found one significant effect: monozygotic pairs who stopped living together later in life were more likely to be similar in having (or not having) DSM-III-R alcohol dependence (odds ratio=1.06, p<0.04; DSM-IV alcohol abuse/dependence, p<0.07; DSM-III-R alcohol abuse/dependence, nonsignificant; DSM-IV alcohol dependence, nonsignificant). We found no evidence that differences in monozygotic and dizygotic pairs’ similarity of diagnostic outcomes were associated with differences in the similarity of their childhood environments or their level of recent social contact.
Analyses of the Epidemiologic Sample
table 1 displays twin pair resemblance among all interviewed male pairs for DSM-III-R and DSM-IV alcohol abuse, alcohol dependence, alcohol abuse/dependence, and a multiple threshold definition (unaffected, abuse only, dependence). Results of chi-square tests suggest that the multiple threshold definition fitted the data well for both DSM-III-R diagnosis (monozygotic twins, χ
2=3.5; dizygotic twins, χ
2=6.2) and DSM-IV diagnosis (monozygotic twins, χ
2=3.5; dizygotic twins, χ
2=4.6; df=5, p>0.05, for all tests), consistent with alcohol abuse and alcohol dependence forming a continuum of severity. For analyses of alcohol abuse, we did not use the hierarchical rule; all subjects who met criteria for alcohol abuse were included, whether or not they also met criteria for alcohol dependence.
The concordances and odds ratios in
table 1 show that the risk of an alcohol-related disorder in the co-twin of an affected twin is increased substantially over the sample prevalence. The odds ratios and pair correlations were significantly higher among monozygotic than among dizygotic pairs, suggesting the importance of genetic influences on variation in risk of these disorders.
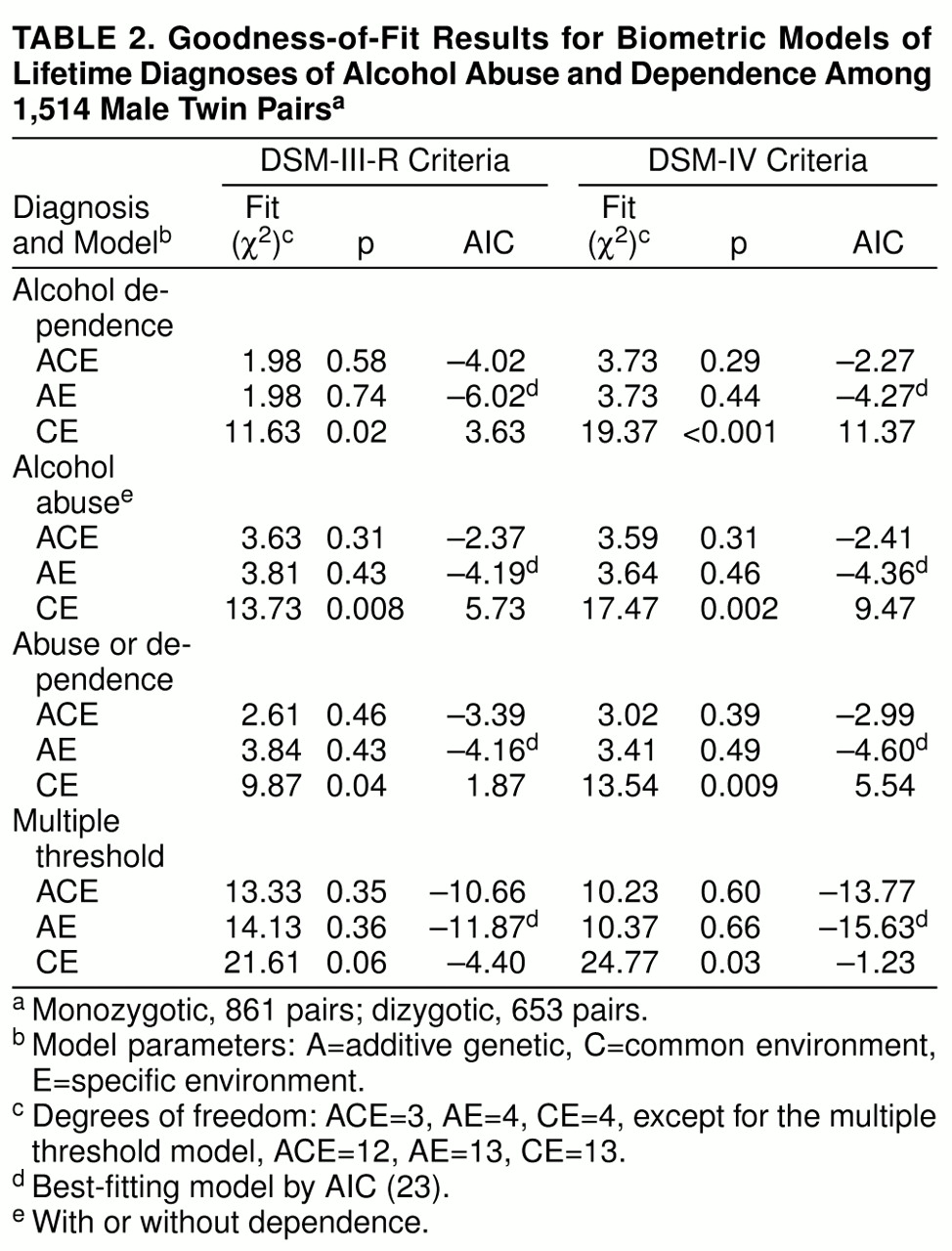
The results in
table 2 show that for each diagnosis, the best-fitting model was AE, which assigns all familial resemblance to genetic sources. There was little evidence that environmental factors shared by siblings influence the development of these disorders. The full (ACE) model had slightly smaller chi-square values than the AE model for alcohol abuse, alcohol abuse/dependence, and multiple threshold, suggesting a small effect of common environment, but the resulting estimates (3%–11%) were not significantly different from 0.
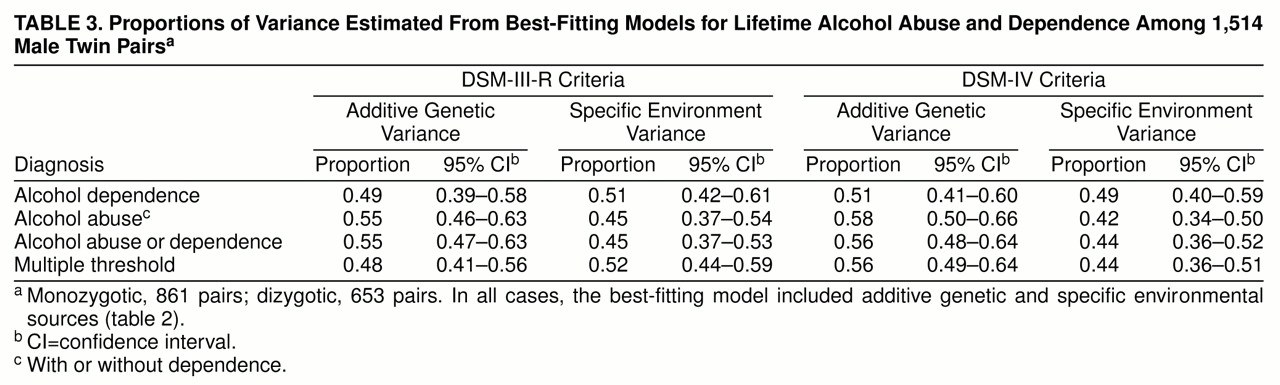
Table 3 displays the standardized proportions of variance estimated from the AE models. For each definition, 48%–58% of the variation in liability is attributed to genetic variation, with the remaining variance attributed to individual specific factors.
We divided the sample into four age groups (18–24, 25–34, 35–44, and 45–56 years) and tested for differences in the model parameters. The 25- to 34-year age group had significantly higher prevalences of alcohol dependence (χ2=20.9, df=3, p<0.001) and alcohol abuse/dependence (χ2=10.7, df=3, p<0.05). However, allowing the genetic and environmental parameters to vary across age groups did not produce a substantial improvement in fit for either diagnosis.
Treatment-Ascertainment Models
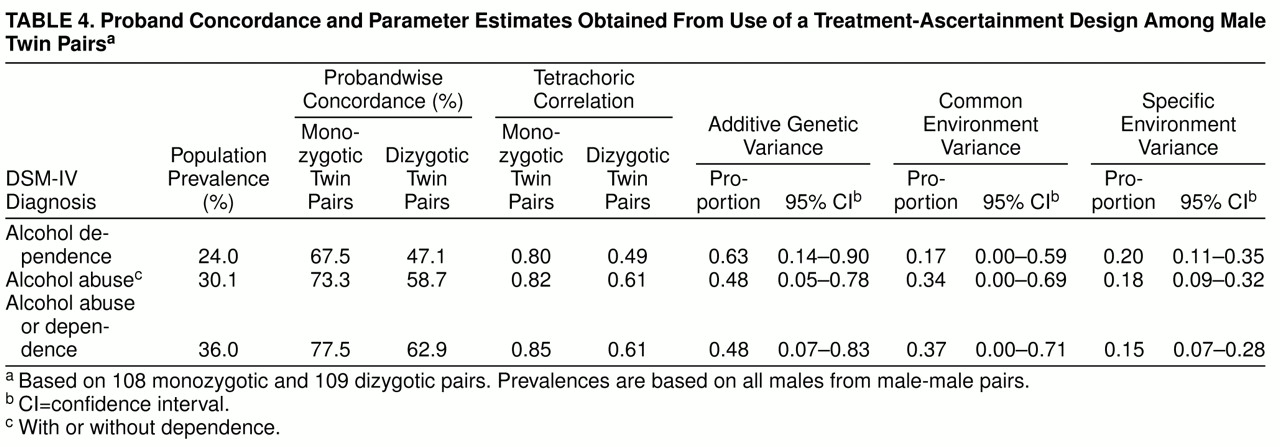
We “simulated” a treatment ascertainment strategy by defining as probands twins who met diagnostic criteria and reported treatment in an inpatient or outpatient alcohol or substance abuse program or who were court-ordered into an outpatient program following an alcohol-related offense. We then simulated a follow-up study by examining the diagnoses of their co-twins.
table 4 shows the probandwise concordances, pair correlations, and resulting biometric estimates for DSM-IV-defined alcohol dependence, alcohol abuse, and alcohol abuse/dependence. (Similar results were obtained for DSM-III-R classifications.) For all three diagnoses, pair resemblance was remarkably strong, but co-twins of affected monozygotic twins were more likely to be affected than co-twins of affected dizygotic twins. Additive genetic estimates were similar to those obtained with the use of the epidemiologic sample, but these analyses also yielded evidence for shared environmental influences, particularly for alcohol abuse and alcohol abuse/dependence.
DISCUSSION
Like other twin and adoption studies of alcoholism in males, we found evidence for substantial genetic influences. Potential genetic mechanisms include loci associated with aldehyde and alcohol dehydrogenase
(25) and a variant of the dopamine D
2 receptor gene
(26). Although these findings have not been consistently replicated
(27), they provide a plausible mechanism for direct genetic influence on clinical phenotypes. Possible mechanisms for indirect genetic transmission include personality traits
(28) and comorbid psychopathology such as affective and conduct disorders
(29). Findings of distinct loci associated with different components of alcohol-related behaviors in animals
(30), research in humans suggesting genetic influences on initial sensitivity to alcoholism
(31), and reports linking alcohol-related phenotypes to multiple chromosomes
(32) underscore the likely etiological complexity of alcoholism.
Genetic Influence and Nosology
Unlike several other studies
(2,
4,
8,
10,
11), but similar to our parallel study of alcoholism in female twins
(33), we found little evidence that genetic factors are more important for the etiology of narrowly defined alcoholism (alcohol dependence or alcohol dependence with tolerance or physiological dependence) than for broadly defined alcoholism (alcohol abuse/dependence or “problem drinking”). These results are consistent with a continuum of alcohol-related problems and do not suggest the existence of separate etiologies for alcohol abuse and dependence. Factor analytic studies of diagnostic criteria have identified multiple dimensions
(34), but these factors differ from DSM-based diagnoses. The results of several family
(35), adoption
(36), and twin
(11) studies suggest etiological heterogeneity for other definitions and subtypes of alcoholism, which merit further investigation.
Ascertainment Method
In contrast to several prior studies, we found little evidence that within-family environmental factors are involved in the development of alcohol-related disorders. The studies that found evidence for common environment were based on treatment samples
(10,
11) or registrations for alcohol-related offenses
(4), in which subjects with the disorders may have been more severely affected or more likely to have comorbid characteristics than the subjects with the disorders in the present study. Previously, we presented analyses of simulated data
(37) showing that shared environmental effects could be mimicked by common environmental processes that influence ascertainment of cases but are unrelated to etiology (e.g., treatment of one twin increasing the probability that the co-twin seeks help, regardless of zygosity). When we applied a treatment ascertainment strategy to the current sample, we obtained similar results. Since the evidence of common environmental effects was stronger for the broader definitions of alcohol abuse and alcohol abuse/dependence than for alcohol dependence, these data suggest that the differences among studies are not due to differences in severity among the subjects but to common environmental effects that influence who is included in treatment-based samples.
Age Effects
Similar to findings in other epidemiologic studies, we observed a nonlinear association between age and lifetime prevalence of alcohol dependence, with the highest prevalences observed among men aged 25–44 years. The lower prevalences in the older group are consistent with estimates from national samples
(38), while those in the youngest group are most likely due to censoring because individuals are still within the age of risk.
Although our tests of equality of genetic and environmental estimates across age groups have limited power, the results suggest that the estimates from our twin models are unlikely to have been substantially biased by age heterogeneity in the sample. This finding is similar to our recent study of temperance board registration among Swedish male twins
(4), which found no evidence of cohort differences in genetic and environmental influences.
Limitations
This study was limited to male Caucasian twins born in Virginia and may not be representative of individuals from other regions or ethnic backgrounds. Twins have an increased rate of perinatal injury and slightly lower intellectual functioning but are apparently representative in rates of psychopathology
(39,
40).
We attempted to interview all eligible twins, but nonparticipants may have differed from the subjects interviewed. Analyses comparing twins from complete and incomplete pairs do not suggest that the twins excluded from twin pair analyses differed in prevalence of alcohol abuse or dependence, but other biases are possible.
The use of telephone interviews rather than in-person interviews may have increased the unreliability of our assessments. Interviewers may be better able to perceive and correct misunderstandings of questions if they have visual cues from respondents. However, some evidence suggests that the more anonymous format of a telephone interview may improve validity by increasing willingness to discuss socially undesirable behaviors
(41).
We found that pair similarity in DSM-III-R alcohol dependence was higher for monozygotic twins who separated later in life. This could represent a violation of the equal environment assumption, suggesting an overestimate of genetic influences on this disorder. It is also plausible that early separation is an outcome (rather than a cause) of differences in alcohol-related behavior, and this would not be a violation of the equal environment assumption.
The lifetime prevalences of alcohol dependence in this sample (by DSM-III-R criteria, 27.4%; by DSM-IV criteria, 24.0%) were somewhat higher than those for males of similar age from national epidemiologic studies, including the Epidemiologic Catchment Area Study (by DSM-III-R criteria, 25.1%)
(38), the National Comorbidity Survey (by DSM-III-R criteria, 20.7%)
(42), and the National Longitudinal Alcohol Epidemiologic Survey (by DSM-IV criteria, 21.7%)
(43). A partial explanation may be that our sample included fewer abstainers than did other studies. The prevalence of DSM-IV alcohol dependence was 27.6% among male drinkers aged 18–54 years in the National Longitudinal Alcohol Epidemiologic Survey, comparable to 27.1% among drinkers in our sample (defined as individuals who had 12 or more drinks during any year). When we applied a more stringent diagnostic definition (requiring subjects to meet four of nine DSM-III-R criteria), the prevalence was 19.5%. The resulting pair correlations (for monozygotic twins, r=0.57; for dizygotic twins, r=0.26) and heritability (57%) were similar to those we report for other definitions, suggesting that the breadth of our definition did not alter the biometric results.
Despite these limitations, this sample arguably provides the best genetically informative data from which to generalize about the etiology of alcohol abuse and dependence among men in the U.S. population. Prior genetically informative studies of alcoholism in males used subjects from foreign countries or subjects identified through treatment or alcohol offenses.