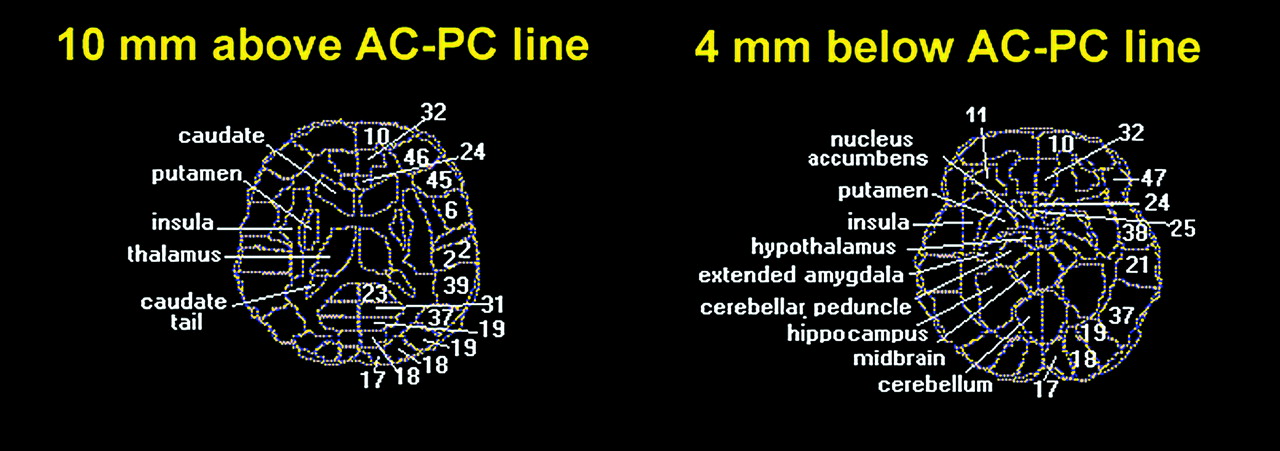
Despite agreement within the functional imaging literature on the importance of the anterior cingulate as a predictor of therapeutic response, studies that attempt to localize the affected area more precisely and explore functionally related brain areas are needed. Since partial volume effects, inaccuracy in region of interest placement, and use of large medial region of interest boxes for activity assessment may average together or dilute ventral anterior cingulate effects, variation in the location and direction of anterior medial activity may be prominent. In the current study we extended our data analysis, using statistical probability mapping and a larger number of subjects to increase statistical power.
DISCUSSION
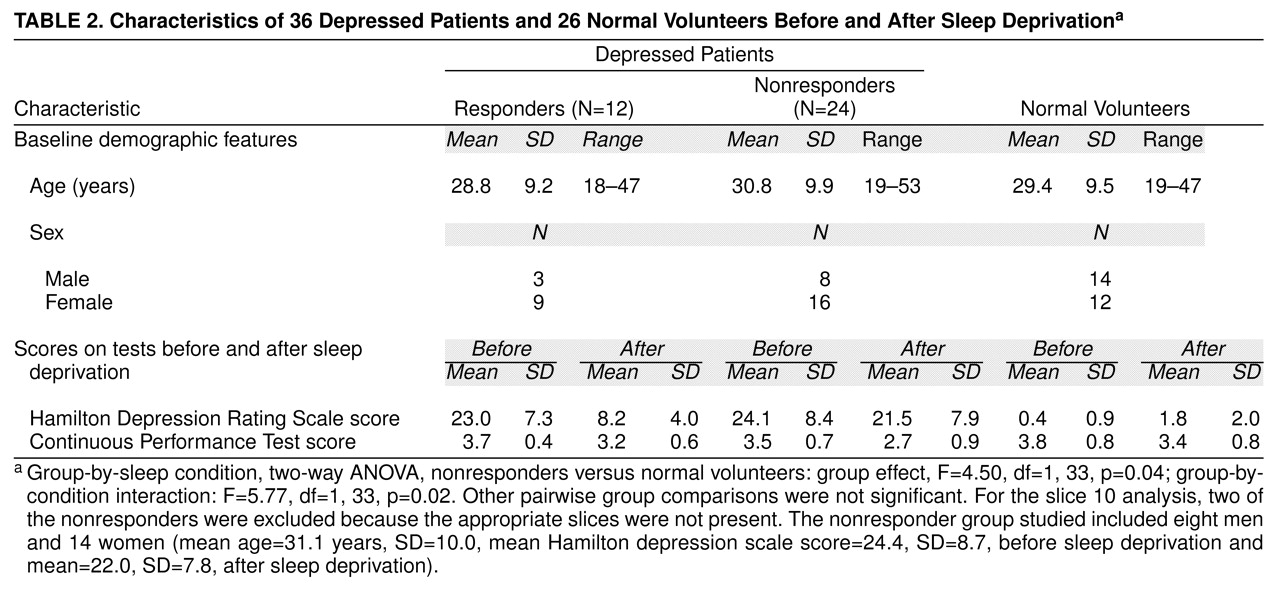
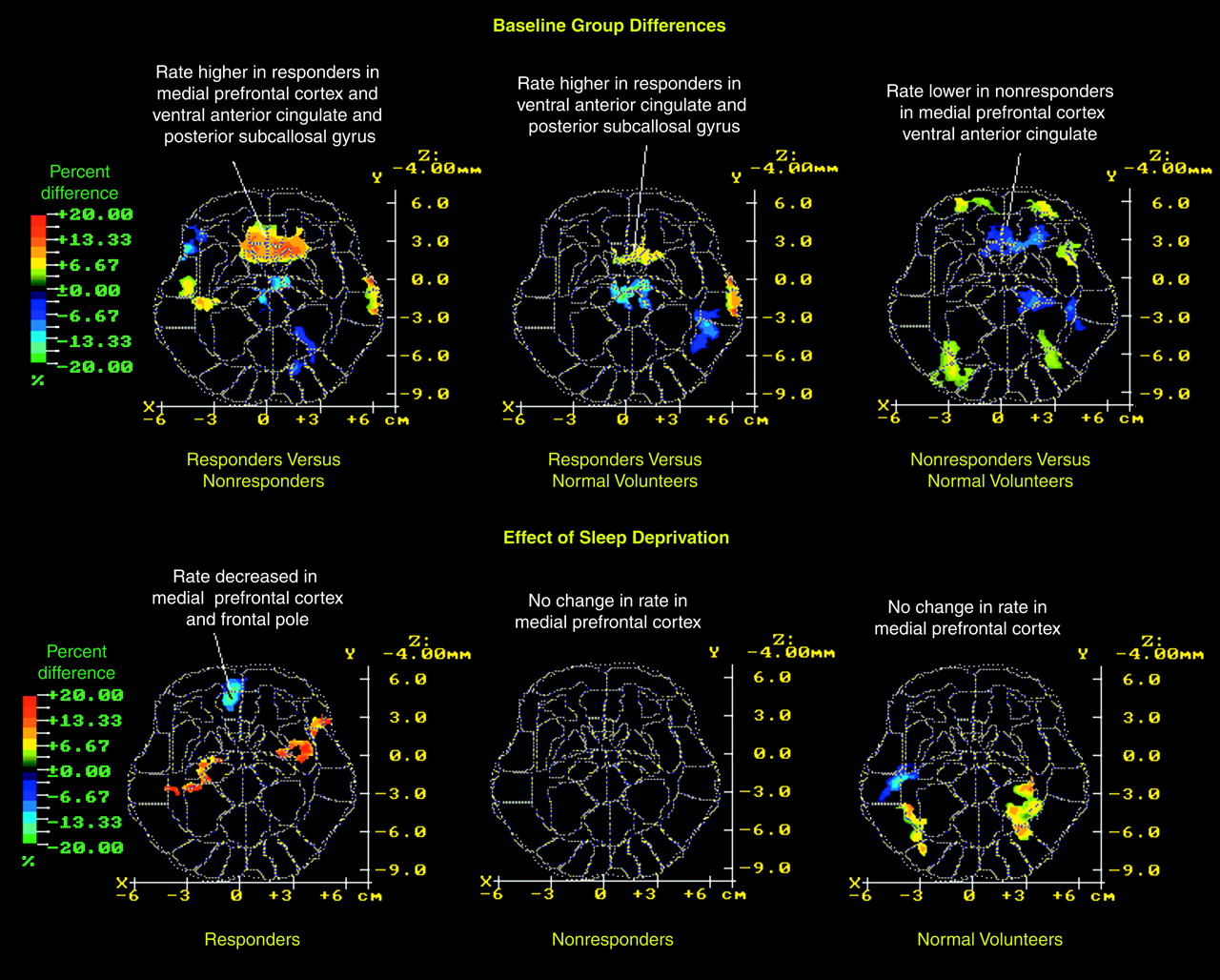
This study has confirmed and extended two major findings from our earlier study
(7). First, depressed patients who showed a favorable clinical response to sleep deprivation had higher relative metabolic rates in the ventral anterior cingulate, medial prefrontal cortex, and posterior subcallosal cortex at baseline than either normal volunteers or depressed patients who did not respond to sleep deprivation. Second, metabolic activity in the medial prefrontal cortex and frontal pole decreased and normalized in responders after sleep deprivation but not in nonresponders and normal volunteers, who remained unchanged. Findings in the 21 new patients confirmed findings in the 15 earlier-studied patients
(7) and are consistent with reports by other investigators who used single photon emission computed tomography to study sleep deprivation
(6,
8,
9).
Do the metabolic correlates of the antidepressant response to sleep deprivation (relatively high metabolism in the ventral anterior cingulate at baseline and decreased metabolism in the medial prefrontal cortex after clinical improvement) also characterize response to other forms of treatment such as antidepressant medications, ECT, light therapy, and cognitive therapy? What is the role of the anterior cingulate in affective disorder and in normal affective function?
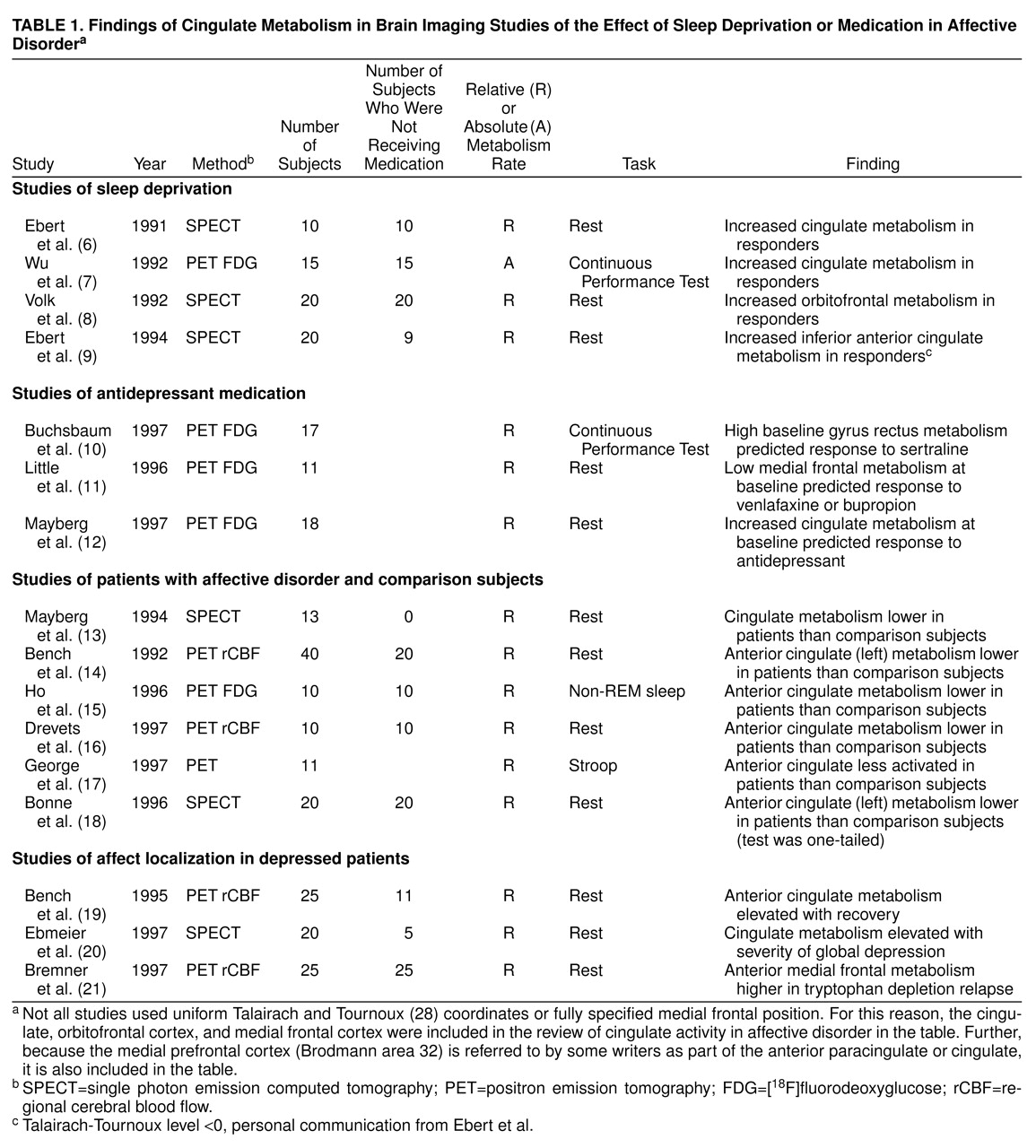
Table 1 summarizes several studies on the effects of antidepressant medication. Buchsbaum et al.
(10) reported a significant correlation between extent of improvement measured by the Hamilton depression scale and change in metabolic rate in the anterior cingulate gyrus (summed across its extent) in patients treated with sertraline. Increased relative metabolism in the adjacent and slightly inferior region, at x=–10, y=16, z=–12 (labeled “Brodmann area 25” [ventral cingulate] in Talairach and Tournoux
[28] and “rectal gyrus” in Matsui and Hirano
[27]), also predicted clinical response to sertraline (r=0.76), a finding with an effect center only about 14–19 mm distant from the prediction area in the current data.
Similarly, Mayberg et al.
(12) found that patients with unipolar depression who had high pretreatment metabolism in the rostral anterior cingulate later proved to be responders to treatment with unspecified antidepressants. By contrast, Little et al.
(11) reported that low metabolism in the cingulate gyrus predicted antidepressant response to venlafaxine or bupropion. The discrepancies between studies could reflect differential effects of the antidepressant treatments studied, differences in anatomic localization, or differences in dorsoventral level. Differing gender compositions of the various study groups may be another confounding variable.
The studies listed in
Table 1 generally support a role for the cingulate in affect regulation. Cingulate activity is usually lower in untreated depressed patients than in comparison subjects and correlates with mood change in studies of depressed patients.
The anterior cingulate and medial prefrontal cortex are believed to modulate internal emotional response. Devinsky et al.
(33) distinguished the affect-related role of rostral areas 24 and 25 from the cognitive role of caudal Brodmann area 24
and Brodmann area 32. Note that high metabolic activity at baseline in responders is found in both affective (Brodmann areas 24 and 25) and cognitive (Brodmann area 32) areas. The posttreatment change in metabolic rate in responders to sleep deprivation was confined to cognitive areas (Brodmann areas 32 and 10). Rostral Brodmann area 32 is connected to the amygdala and parts of the autonomic brainstem nuclei
(33); this differential effect suggests that cognitive factors could be important in the modulation of emotion.
We
(15,
34,
35) and others (reviewed in reference
35) reported reductions in relative frontal lobe to occipital lobe metabolism in patients with unipolar and bipolar depression while awake and in patients with unipolar depression during non-REM sleep. Decreased frontal lobe metabolism has also been reported in depression associated with conditions such as Parkinson’s disease
(36,
37), Huntington’s disease
(38), and seizure disorders
(39). In the current study, sleep-deprived normal volunteers showed decreased metabolism in the lateral prefrontal cortex but increased metabolism in the occipital cortex (
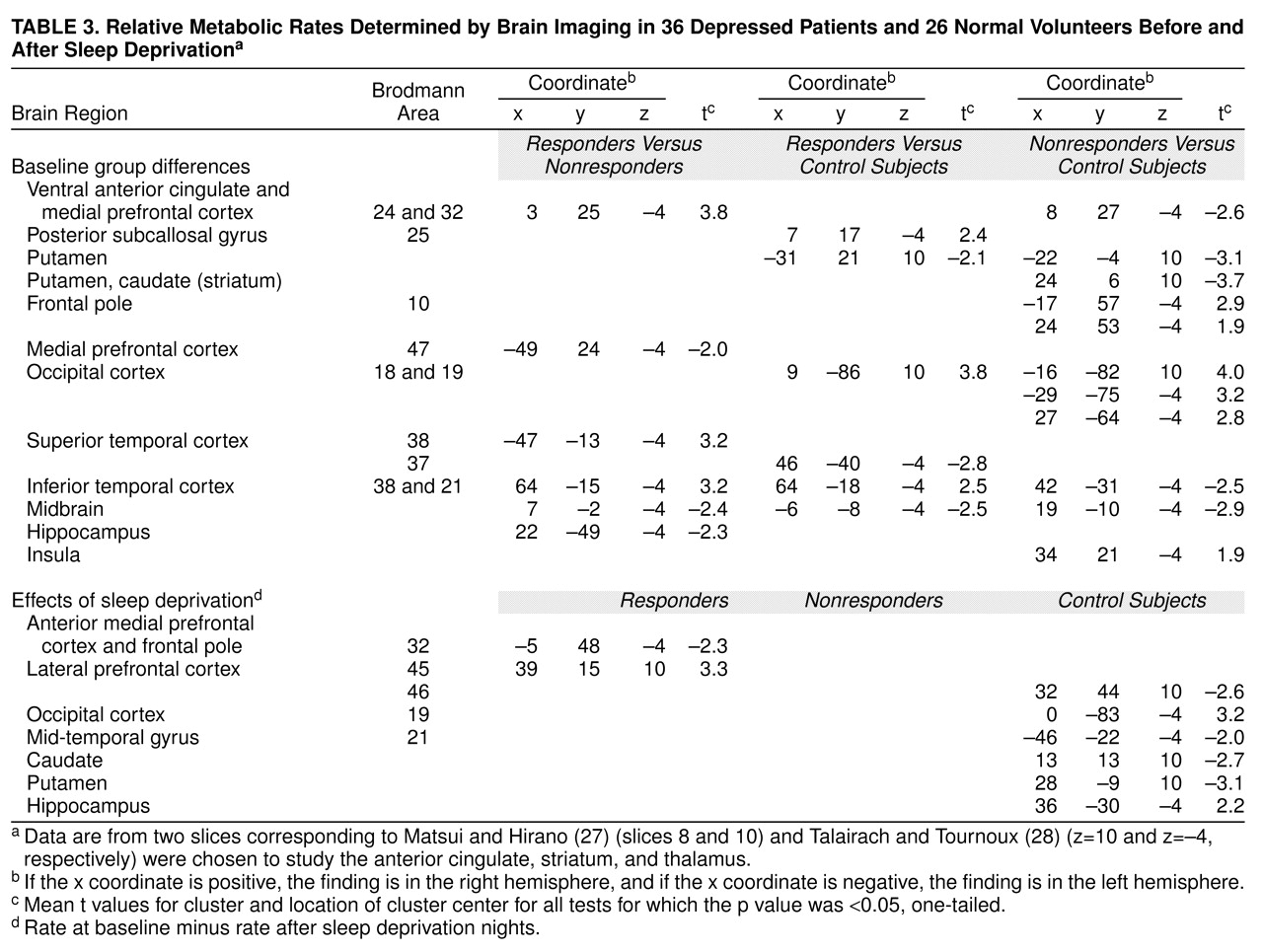
Table 3), a pattern dissimilar to that seen in depressed patients who responded to sleep deprivation. Responders had significantly higher metabolism in the bilateral visual association cortex than did normal volunteers at baseline, but they actually showed increased metabolism in the lateral prefrontal cortex after sleep deprivation. Thus, in responders, sleep deprivation seemed to partly correct the pretreatment pattern of relative hypofrontality seen in other studies (reviewed in reference
15).
Note, however, that values for the ventral anterior cingulate (Brodmann areas 25 and 24) were higher in responders than in either nonresponders or normal volunteers at baseline, but sleep deprivation effects tended to be slightly more anterior and located in medial prefrontal Brodmann area 32 rather than Brodmann areas 25 and 24. Posterior subcallosal Brodmann area 25 belongs to an infralimbic circuit that comprises afferent projections from the amygdala, the thalamic paraventricular nucleus, and the hypothalamus
(40). The infralimbic area, which is considered the cortical center of the autonomic nervous system
(41), is involved in the visceral motor response to stressful behavioral and emotional events
(42). This area, which was predictive of therapeutic response to sleep deprivation, corresponds to the posterior subcallosal gyrus or ventral compartment of the model proposed by Mayberg et al.
(12,
43).
In addition to differences in anterior cingulate metabolism, depressed patients had significantly lower striatal and temporal relative metabolic rates than normal volunteers. The striatal finding is compatible with reports that depressed patients have decreased relative basal ganglia metabolism
(15,
44) and perfusion
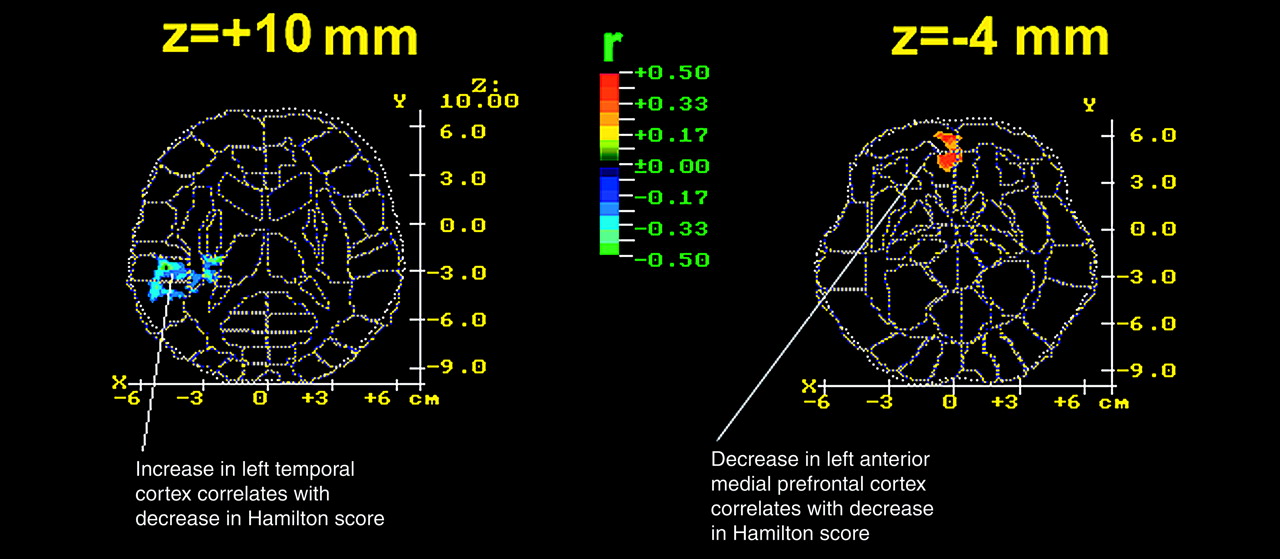
(45). In depressed patients, relative metabolism in the temporal cortex correlated negatively with change in Hamilton depression scale score after sleep deprivation, a finding consistent with the report by Cohen et al.
(46) that depressed patients had decreased metabolism in the temporal cortex relative to values in normal volunteers.
The dopamine system may be implicated in the response to sleep deprivation. Decreased dopamine activity has been hypothesized for depression
(47). Dopaminergic projections from the ventral tegmental area inhibit excitatory thalamic input into the subgenual region. Decreased dopaminergic firing in responders compared with normal volunteers or nonresponders from the ventral tegmental area to the paraventricular thalamic nucleus would result in a disinhibition of thalamocortical firing that would be compatible with increased activity in the infralimbic Brodmann area 25 in the responders. This suggests that responders may be hypodopaminergic compared with nonresponders and normal volunteers.
Ebert et al.
(9) reported that responders had significantly greater displacement of D
2 ligands after sleep deprivation than before but that nonresponders showed no changes. Their finding suggests that dopaminergic function in responders, but not nonresponders, was enhanced by sleep deprivation.
Gerner et al.
(48), in a comparison of CSF samples from depressed patients before and after sleep deprivation, found increased posttreatment levels of the dopamine metabolite homovanillic acid in responders but not nonresponders, suggesting that dopamine activity was associated with clinical antidepressant effects.
Serotonin effects may also relate to differences between responders and nonresponders. High baseline metabolic rates in ventral medial frontal regions predicted clinical response to the selective serotonin reuptake inhibitor sertraline
(10). In that study, as in the current study, metabolic rates in lateral prefrontal regions increased after treatment
(10). A complex interaction between sleep deprivation recovery and the serotonin system is also suggested by serotonin depletion studies
(49). Specific studies of
3H-imipramine and 3H-paroxetine binding, however, differentiated control subjects and depressed suicide victims in the hippocampus but not the cingulate
(50)Our current results confirm our a priori hypothesis about the involvement of the anterior cingulate and the prefrontal cortex in the response to sleep deprivation. Our observations, however, suggest secondary hypotheses to be tested.
First, three other areas were implicated in the antidepressant response. The increase in metabolic rate in the left superior temporal lobe (Brodmann area 37) and right insula correlated significantly with improvement in the depressed group after sleep deprivation. Metabolic activity in the right lateral prefrontal cortex (Brodmann area 45) also increased significantly after sleep deprivation in responders.
Second, the metabolic rate in the putamen, occipital cortex (Brodmann areas 18 and 19), inferior temporal cortex (Brodmann areas 38 and 21), and midbrain was abnormal both at baseline and after sleep deprivation in responders and nonresponders. Despite a 64% improvement in mean depression ratings, responders did not show changes in metabolic rate in these four areas, suggesting that they may not be involved in the pathophysiology or treatment of depressed mood per se. One caveat, however, is that responders remained mildly depressed (Hamilton depression scale mean score=8.4 versus 1.8 for normal volunteers). It would be interesting to determine whether metabolism completely normalizes in these and other areas after full symptomatic recovery.
Third, nonresponders had abnormally low relative metabolic activity in the anterior cingulate at baseline. Many investigators (
Table 1) reported reduced perfusion or metabolism in the anterior cingulate and prefrontal cortex ventral to the genu of the corpus callosum in patients with affective disorders. Since there were more nonresponders to sleep deprivation than responders, this group effect is not surprising. Differences between subtypes of patients with affective disorder based on therapeutic response are likely to involve a network of brain regions, of which the ventral anterior cingulate is only one.