Brain Metabolism in Methamphetamine Users After Short and Protracted Abstinence Intervals
Among the methamphetamine users who were evaluated twice, there were no differences in global metabolism measured after short versus protracted intervals of abstinence (mean=42.6 μmol/100 g per minute [SD=4.0] and 40.8 μmol/100 g per minute [SD=3.4], respectively) or in the absolute metabolic measures in the striatum, thalamus, or occipital cortex. As seen in
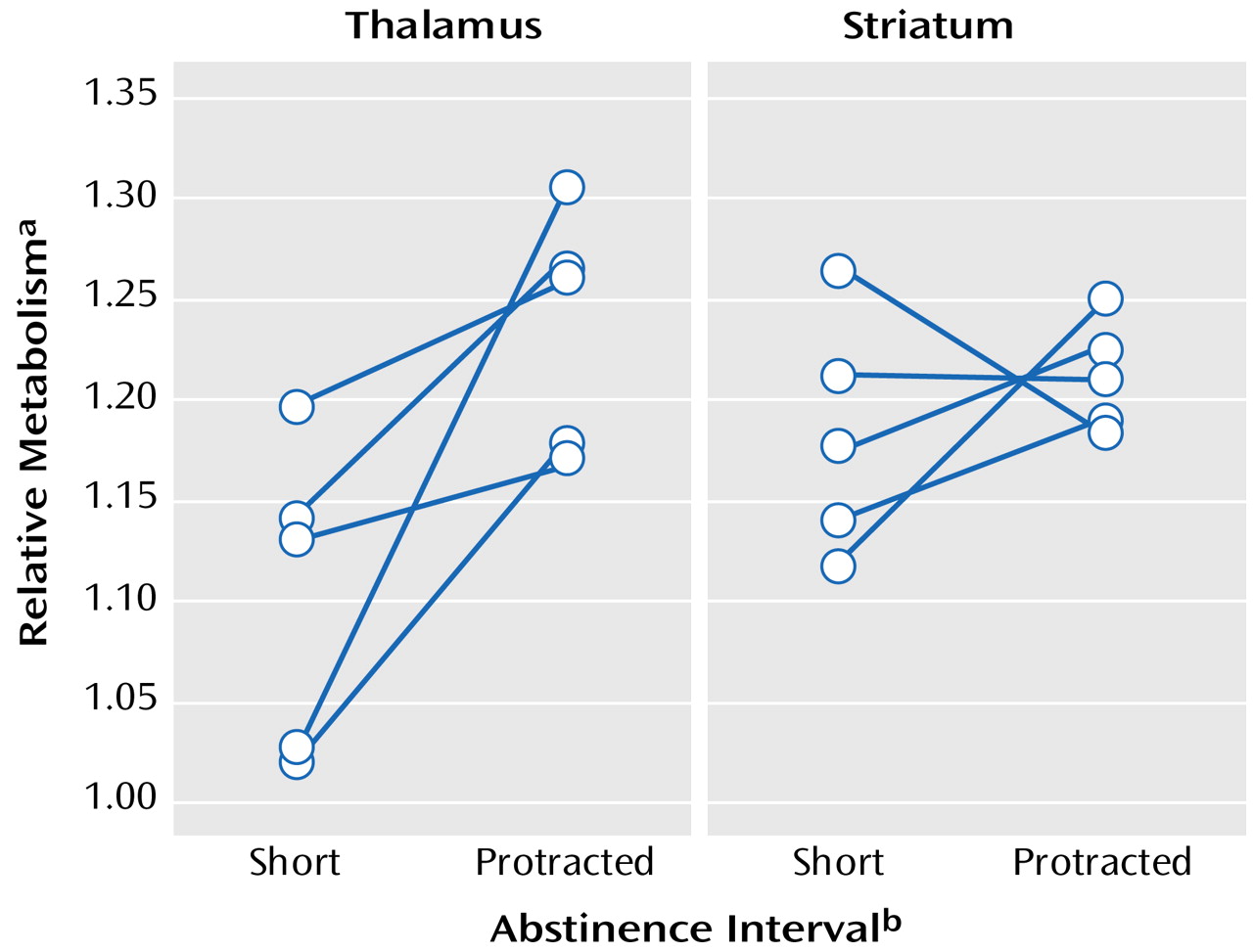
Figure 1, comparison of “relative” metabolism measured after short and protracted intervals of abstinence showed significant increases in thalamic activity after protracted abstinence (mean=12%, SD=9%) (t=2.3, df=9, p<0.05) but no significant changes in the striatum (mean=2.7%, SD=6.7%) (t=1.3, df=9, p<0.23) or occipital cortex (mean=0.4%, SD=9%) (t=1.2, df=9, p<0.28).
As seen in
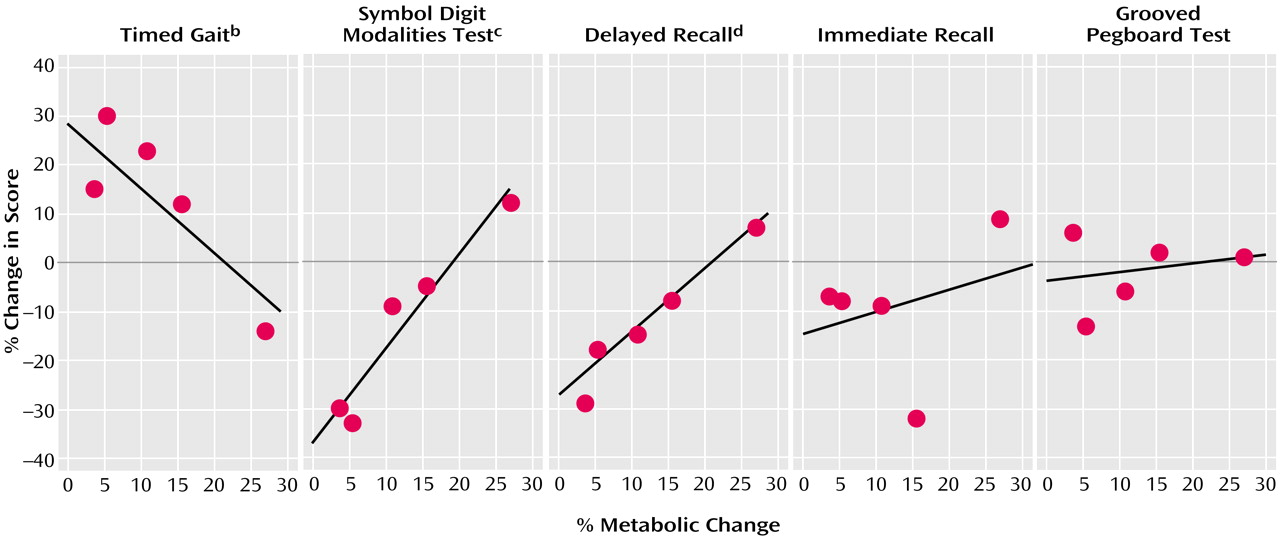
Figure 2, analysis of the correlation between thalamic activity changes and changes in performance on neuropsychological tests for which we hypothesized an association with metabolic changes showed a significant association for timed gait, Symbol Digit Modalities Test, and delayed recall but not with the changes in the grooved pegboard task (r=0.26, df=3, p=0.68) or the immediate or interference recall (r=0.32, df=3, p=0.60).
Comparison of Healthy Subjects With Methamphetamine Users Tested After Short and Protracted Abstinence Intervals
Absolute global brain metabolism was not significantly different in the methamphetamine abusers tested either after a short interval of abstinence (mean=38.5 μmol/100 g per minute, SD=8.5) or after protracted abstinence (mean=37.3 μmol/100 g per minute, SD=6.3) than in the comparison subjects (mean=33.8 μmol/100 g per minute, SD=7.4).
Regional changes were assessed by using the regional values normalized by the global measures. Compared with the relative metabolic measures seen in the striatum of the healthy subjects (caudate: mean=1.35, SD=0.06; putamen: mean=1.41, SD=0.09), the relative metabolic measures of the methamphetamine abusers were significantly lower after both a short abstinence interval (caudate: mean=1.2, SD=0.1 [t=–4.4, df=21, p<0.0002]; putamen: mean=1.31, SD=0.08 [t=–2.8, df=21, p<0.01]) and after protracted abstinence (caudate: mean=1.23, SD=0.09 [t=–4.4, df=22, p<0.0002; putamen: mean=1.29, SD=0.1 [t=–3.1, df=22, p<0.006]). Relative metabolism in the thalamus was significantly lower in the methamphetamine abusers after a short abstinence interval (mean=1.11, SD=0.08) relative to the comparison subjects (mean=1.31, SD=0.10) (t=–5.3, df=21, p<0.0001); however, thalamic metabolism in the methamphetamine abusers after protracted abstinence (mean=1.23, SD=0.09) did not differ from that of the comparison subjects.
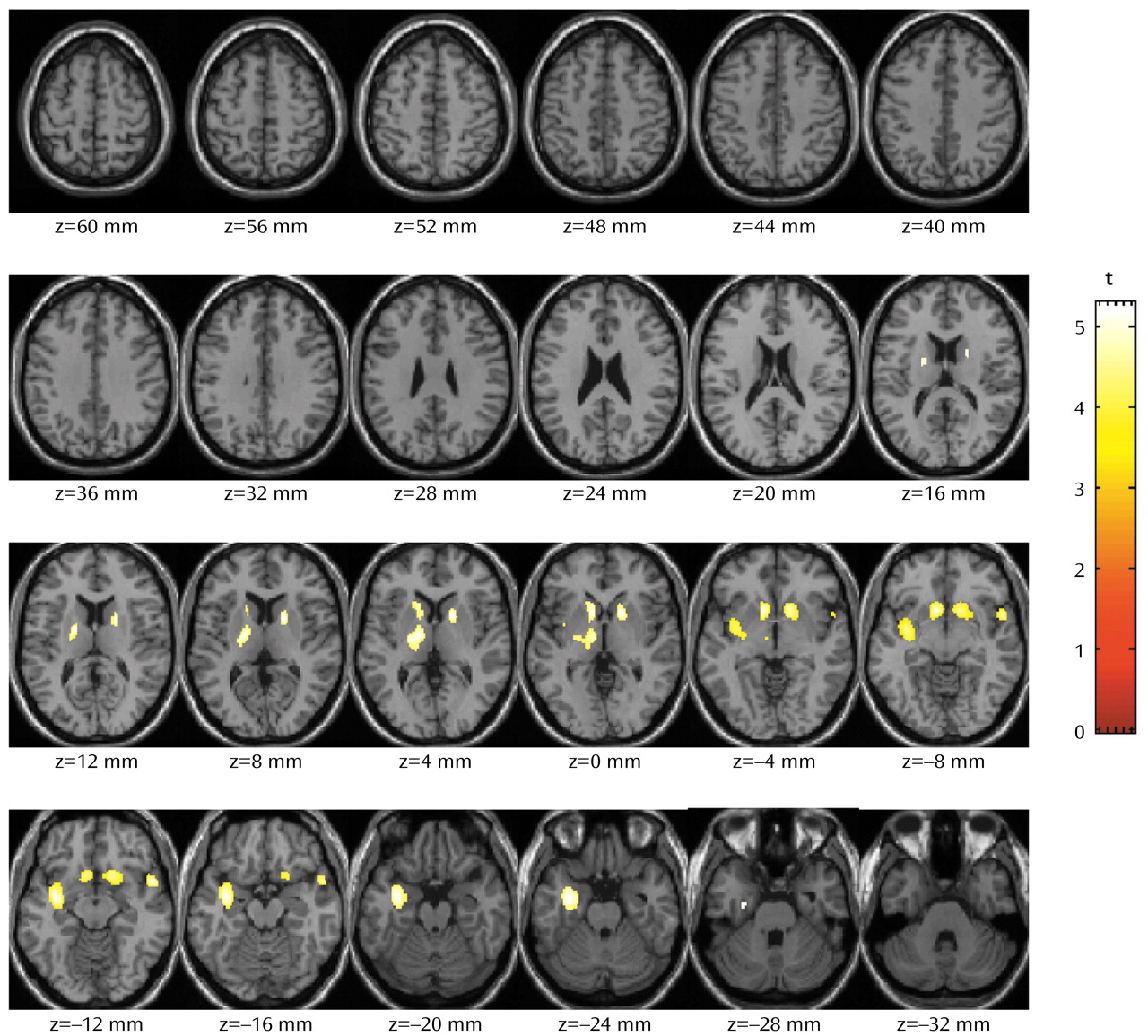
The statistical parametric mapping analyses of the absolute metabolic values showed no differences between groups. The normalized metabolic values, with smaller intersubject variability, were also analyzed by using statistical parametric mapping, and the results were similar to those obtained with the region of interest method. The statistical parametric mapping analyses (
Figure 3) yielded a large contiguous cluster (4,440 voxels) that encompassed three subclusters (left subcortical nuclei [including dorsal striatum, nucleus accumbens, and thalamus]: –8, 14, –6; left insula: –34, –6, –12; and right striatum: 14, 14, –6). The significance of this cluster is p<0.0001 (corrected at the cluster level).
For the region of interest method, metabolism in striatum was significantly lower in the methamphetamine abusers after both a short abstinence interval and protracted abstinence than in the comparison subjects. Similarly, the statistical parametric mapping analyses revealed significant decreases in the nucleus accumbens in the methamphetamine abusers whether they were tested after a short or protracted interval of abstinence. In contrast, thalamic metabolism, which was significantly lower in methamphetamine users after a short abstinence interval, was not different in the methamphetamine users after protracted abstinence relative to the comparison subjects. In addition, statistical parametric mapping revealed that relative to the comparison subjects, methamphetamine abusers had decreased metabolism in the left insular cortex. The decreases in insular metabolism were more accentuated in the methamphetamine abusers tested after a short than after a protracted interval of abstinence.