Heavy alcohol consumption can cause serious health problems, morbidity, and death (
1). Finding efficacious treatments that can decrease the severity of drinking in alcohol-dependent individuals is therefore an important scientific and health goal. Because the serotonin (5-HT) system is an important regulator of the severity of alcohol drinking (
2), medications that affect the function of the 5-HT transporter (5-HTT), which plays an important role in the regulation of neuronal 5-HT function (
3), appear particularly promising (
4).
SLC6A4, which is located on chromosome 17q11.1–q12, is the only known gene encoding the 5-HTT in the human genome (
5). Because the amino acid sequence and sensitivity of the human 5-HTT are common to all tissues, including blood cells and neurons (
5,
6), it is reasonable to suspect that genetic variation that alters the function of the platelet 5-HTT might also be seen in neuronal cells.
The
SLC6A4 promoter contains a functional polymorphic region (5′-regulatory region of the 5-HTT; 5′-HTTLPR) with a long form (L) that possesses an additional 44 base pairs that are absent in the short variant (S). The 5′-HTTLPR polymorphism has been investigated extensively for a possible association with a wide range of psychiatric disorders. For example, the S allele has been associated with anxiety-related personality traits (
7,
8) and depression (
9), while the L allele has been linked with obsessive-compulsive disorder (
10) and a better antidepressant response in depression (
11). Variants of the 5-HTT gene also have been associated with substance use disorders, including alcohol dependence (
12). Thus, 5′-HTTLPR polymorphisms can influence various psychiatric conditions, including alcohol dependence.
In healthy individuals, the LL genotype, compared with the LS and SS genotypes, has been associated with higher transcription rates in lymphoblast cells, greater 5-HT uptake into platelets (
13) and lymphoblasts (
14), and greater binding of [123I]2-β-carboxymethoxy-3-β-(4-iodophenyl)tropane (β-CIT) in raphe nuclei (
15). By contrast, neuroimaging studies have shown that alcohol-dependent individuals with the LL genotype, compared with their healthy counterparts, have lower β-CIT neuronal binding to 5-HTTs in the raphe nuclei (
15). Consistent with these findings in neuronal tissue, studies in platelets have shown that alcohol-dependent individuals with the LL genotype, compared with those with the SS genotype, have significantly less 5-HT uptake and reduced paroxetine binding capacity (
3,
16). Also, among those with the L variant, greater severity of lifetime drinking of alcohol has been associated with lower levels of 5-HT uptake and binding (
3). These findings have suggested a gene-by-environment interaction whereby 5-HTT gene expression is suppressed by increased alcohol consumption via an unknown mechanism, which in turn perpetuates further consumption (
3). Thus, LL genotype in alcohol-dependent individuals has an important effect on the function of the 5-HTT and the severity of lifetime drinking.
We discovered that another functional allelic variant, the single-nucleotide polymorphism (SNP) rs1042173 (T/G) in the 3′-untranslated region (3′-UTR) of the 5-HTT gene, can be associated with drinking severity. TT homozygotes, compared with G carriers, had a significantly higher drinking severity (
17). Significantly, T-allele-transfected HeLa cells, compared with their G-allele counterparts, had lower expression levels for both 5-HTT mRNA and protein (
17). Because the rs1042173 SNP is located at or near a potential binding site for several microRNAs (
18), and variation at this location may alter expression levels by affecting the stability of mRNA (
19,
20), it would be reasonable to propose that the TT genotype of the 3′-UTR SNP might increase the effect of the LL genotype of the 5′-HTTLPR to produce a more marked reduction in 5-HTT expression in alcohol-dependent individuals. Lowered 5-HTT expression and binding would paradoxically be associated with reduced rather than increased 5-HT intrasynaptic neurotransmission and with up-regulation of postsynaptic 5-HT receptors (
4). This is because 5-HTTs in the raphe nuclei are somatodendritic, and therefore auto-regulatory mechanisms would have the effect of decreasing 5-HT firing rates (
21) and up-regulating postsynaptic 5-HT receptors.
This relative hyposerotonergic state with up-regulation of postsynaptic 5-HT receptors might explain why alcohol-dependent individuals with the LL genotype of the 5′-HTTLPR, compared with those with the LS/SS genotype, have a greater urge to use alcohol (
22). This supports the prediction from our hypothesis that blockade of these up-regulated postsynaptic 5-HT receptors in alcohol-dependent individuals with the LL genotype of the 5′-HTTLPR by ondansetron would result in a marked reduction in the severity of drinking (
4). Other plausible explanations for ondansetron's antidrinking effects include the blockade of ethanol-induced receptor sensitization in the 5-HT system (
23), which we would expect to be greater in individuals with the LL compared with the LS or SS genotype because of decreased expression of the 5-HTT gene (
15).
Predicated on the hypothesis that possession of the TT genotype of rs1042173—like the LL genotype of the 5′-HTTLPR in alcohol-dependent individuals—suppresses 5-HTT expression and binding (albeit through different molecular mechanisms), it is reasonable to hypothesize further that ondansetron's therapeutic effect would be greatest among alcohol-dependent individuals who possessed the combination of the LL and TT genotypes. We therefore tested two predictions of our hypotheses. First, ondansetron would have a greater effect of reducing the severity of alcohol drinking (as measured in drinks per drinking day, our primary efficacy variable) and increasing the percentage of days abstinent (our secondary outcome variable) among alcohol-dependent individuals with the LL genotype compared with S carriers of the 5′-HTTLPR. Second, ondansetron's therapeutic effect would be greatest among alcohol-dependent individuals who possessed both the LL genotype of the 5′-HTTLPR and the TT genotype of rs1042173 in the 3′-UTR.
To test these hypotheses, we conducted a phase 2, 11-week randomized controlled trial of ondansetron in 283 alcohol-dependent individuals who all received weekly standardized cognitive-behavioral therapy (CBT) as their psychosocial treatment. Prospectively, randomization was stratified at enrollment by 5′-HTTLPR genotype, with additional genotyping for rs1042173 in the 3′-UTR and expression analysis for the 5-HTT gene after randomization.
Method
Participants
The 283 treatment-seeking participants were mostly white males (73.1% were male; 84.8% were white, and the remainder were Hispanic) with alcohol dependence but no other DSM-IV axis I diagnosis other than nicotine dependence (
24). They ranged in age from 20 to 78 years (mean=44.7, SD=12.3). All scored >8 on the Alcohol Use Disorders Identification Test (
25), which assesses the severity of alcohol-related problems. Most participants were enrolled at the University of Texas Health Science Center at San Antonio (N=211) and the remainder at the University of Virginia (N=72). Enrollees were current drinkers of alcohol; they were not abstinent and were not withdrawing from alcohol. Enrollees were required to present themselves for clinic attendance with a breath alcohol concentration below 0.02% to complete the rating scales; this limit was achieved without significant withdrawal symptoms, as indicated by the low average values for measures of both breath alcohol concentration and withdrawal symptoms reported in the Results section. Enrollees were physically healthy, were not pregnant, and were not using drugs of abuse at enrollment. All participants provided informed consent.
We used an urn procedure (
26) after the screening visit to randomly assign participants to either treatment or placebo, under each of three different genotypes: the LL, LS, and SS genotypes of the 5′-HTTLPR. Genotyping and analysis of the cohort for rs1042173 (TT, TG, or GG) were performed after randomization.
Study Procedures
Testing took place at the University of Texas Health Science Center at San Antonio from November 14, 2000, to December 17, 2004, and at the University of Virginia from May 13, 2006, to October 20, 2008.
At enrollment, we recorded self-reported alcohol consumption based on the timeline follow-back method over the past 90 days (
27) and performed genotyping on all samples. Other screening parameters, including those of physical health, breath alcohol concentration, and additional psychosocial measures associated with drinking behavior, were the same as detailed in our previous trials (
28,
29).
After randomization, participants entered a single-blind placebo period for 1 week and then received their double-blind medication—either ondansetron at 4 μg/kg of body weight twice daily or placebo—from weeks 2 through 12.
Throughout the trial, participants received weekly assessments of alcohol drinking using the timeline follow-back method, adverse events using the Systematic Assessment for Treatment Emergent Events (
30), and alcohol withdrawal using the revised Clinical Institute Withdrawal Assessment for Alcohol scale (
31). Each weekly assessment also included urine drug testing, collection of data on concomitant medication use, and pill counts.
All participants received weekly standardized, manual-driven CBT (
32,
33) in groups of up to eight. CBT is an integration of cognitive, behavioral, and social learning theory that enables alcoholics to achieve and maintain abstinence by enhancing their ability to manage high-risk situations that can trigger alcohol-seeking behavior (
34,
35). CBT was delivered by skilled master's- and doctoral-level psychologists. The same manual was used at both study sites. Sessions were audiotaped with the permission of study participants. A doctoral-level psychologist supervised CBT delivery by reviewing a random selection of about 10% of the audiotapes. The CBT supervisor—the same individual at both sites throughout the study—met weekly with the psychologists who delivered the treatment, and all those who delivered CBT at both sites were reevaluated formally every 6 months. A review of the supervisor's notes on CBT delivery revealed no evidence of any potential differential “drift” at either site. These stringent procedures for CBT delivery ensured that it was a stable platform of psychosocial treatment against which to measure the added effect of the medication. At scheduled intervals, other measures, including those of physical health and other psychosocial measures, were collected as described previously (
28,
29).
DNA Extraction and Genotyping
Genomic DNA was extracted from the blood of each participant at baseline with a Gentra Puregene kit (QIAGEN Inc., Valencia, Calif.). The 5′-HTTLPR L/S alleles were determined as described previously (
3,
36) with custom-made primers. The alleles of rs1042173 were genotyped with premade TaqMan genotyping assays (Applied Biosystems, Foster City, Calif.) as reported previously (
17). Detailed information on primer/probe sequences and biological information on the two polymorphisms are summarized in
Table 1. To test for potential population stratification between the treatment and placebo groups, we performed genotyping for 24 ancestry-informative markers with all participants' DNA samples to calculate percent ancestry (
Figure 3). These widely used markers have been demonstrated to have high-frequency differences for South American/European ancestry and European/West African ancestry (
37,
38). Information on these markers is provided in Table S1 in the data supplement that accompanies the online edition of this article.
Assessment of sample admixture between treatment groups.
The software program Structure (
http://pritch.bsd.uchicago.edu/software/structure2_2.html) was used to assess population stratification and to estimate genetic ancestry proportions for each participant. We then used the individual ancestry proportion estimates as covariates in all statistical models. Before obtaining the individual ancestry proportion estimates for our population, we assessed the number of parental populations (
K) that captured most of the stratification in the population. For this, we analyzed the data set with K=2 through K=10, and the simulation parameters were set to 10,000 burn-ins and 10,000 Markov chain Monte Carlo iterations. The K value with the highest probability of capturing stratification was 3; hence, we obtained the ancestry proportion estimates by analyzing the data set assuming three parental populations (K=3) and the presence of population admixture. Self-reported ethnicity was not used as additional data in Structure analyses.
Statistical Power and Analyses
Statistical power.
We did our power analysis as described in section 8.3 of Cohen (
39). For our primary outcome variable, drinks per drinking day, a total sample size of 280 (140 in each treatment arm) had a statistical power of 0.91 to detect an effect size of 0.25 in the interaction between treatment and genotype (LL versus LS/SS) at a significance level of 0.05. Similarly, we had a statistical power of 0.91 to detect an effect size of 0.25 in the interaction between 5′-HTTLPR and rs1042173 genotypes. Statistical power was 0.91 to detect an effect size of 0.35 in the effect of combined genotypes and treatment. We therefore had adequate statistical power to test both predictions from our primary hypothesis.
Data quality and statistical analysis.
A database coordinator and statistician supervised data quality. Individual subject plots were checked for unusual values and for completeness. Because the primary efficacy variable, the weekly average of drinks per drinking day, is a direct quantification of the average amount of drinking done per drinking day within each week, it is a valid measure of the severity of drinking. Additionally, we have shown that the differential effects of the 5-HTT promoter and 3′-UTR regions are sensitive to alterations in drinks per drinking day as the measure of drinking severity (
3,
17). The use of drinks per drinking day to measure drinking severity thus has both construct and experimental validity. We validated the calculation of drinks per drinking day as correct against the case records. Although not specific to our primary hypothesis, we chose a secondary outcome variable, percentage of days abstinent, to provide additional clinical support for our findings. Database checks for percentage of days abstinent were the same as those used for drinks per drinking day. Data were analyzed using SAS, version 9.1 (
40), according to the intent-to-treat principle; all participants were included in the analysis.
Our analytic procedure was to use mixed-effects linear regression models, which can accommodate missing data at random, to study the effect of treatment and genotypes, as well as their interaction, on the efficacy measure of drinking. The models included random intercept and slope (for temporal trend) and were adjusted for covariates such as the participants' average drinking levels prior to the study, age, gender, and ethnicity as defined by ancestral markers (i.e., white and Hispanic). For ease of interpretation, we used two separate mixed-effects models to study the interaction between treatment and the two functional variants. In the first model, all two-way interaction terms between genotypes (LL versus LS/SS; TT versus TG/GG) and treatment were included to evaluate the difference in mean values between the genotypes and treatment combinations of interest. In the second model, we compared the ondansetron LL/TT participants with those who had one of the three other genotypes (LL/G carriers, S carriers/TT, or S carriers/G carriers) and tested the hypothesis that ondansetron's therapeutic effect could be greatest among those possessing the LL/TT genotype.
In our analyses, we assumed that data not present were missing at random for the outcomes of interest (drinks per drinking day and percentage of days abstinent). Under that assumption, mixed-effects models would yield consistent results and are the preferred method of handling dropout, compared with other approaches, such as last observation carried forward (
41). While missing at random is not testable directly (
42), we investigated the relationship between dropout and longitudinal drinking outcomes by a joint random-effects model (
43) to simultaneously describe repeated measures of the weekly average of drinks per drinking day and percentage of days abstinent (by the mixed-effects model described above) and time to dropout (by a Cox model). The random effects (random intercept and random slope) in the mixed-effects model were included in the Cox model to link the two models. The estimation was implemented in SAS Proc NLMIXED (
44,
45). We found that after adjusting for risk factors, the time to dropout did not depend on the random effects from the longitudinal model of drinks per drinking day (p=0.89) and percentage of days abstinent (p=0.99), suggesting that dropout time is not correlated with heterogeneity in the weekly average of drinks per drinking day or percentage of days abstinent repeated measures, that is, dropout was not informative. The parameter estimates for the longitudinal outcomes from this joint model were similar to those in the present model, which assumed that data were missing at random. This additional analysis provided evidence of the validity of the missing-at-random assumption.
In all statistical models tested here, we used a dominant genetic model based on the functionality of 5′-HTTLPR and rs1042173 alleles from previous studies as described in the introductory paragraphs of this article; we dichotomized the sample into LL versus S carriers and TT versus G carriers for the 5′-HTTLPR and the SNP rs1042173, respectively.
Results
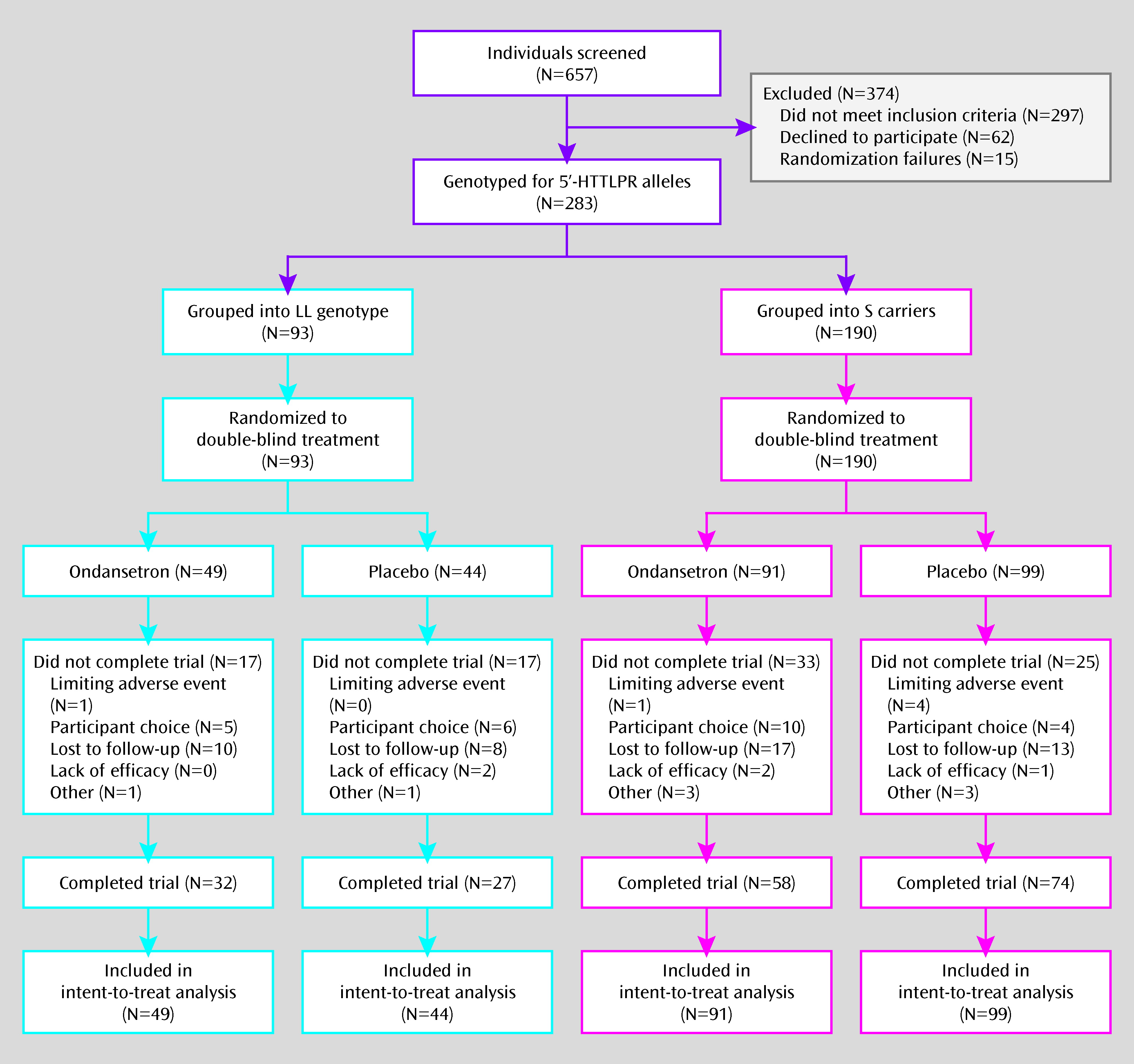
Participants' demographic characteristics did not differ significantly by treatment group for either of the 5′-HTTLPR genotype subgroups (
Table 2). The study population satisfied Hardy-Weinberg requirements (
Table 1). Also, 66.4% of subjects completed all 12 weeks, with no significant group differences (
Figure 1).
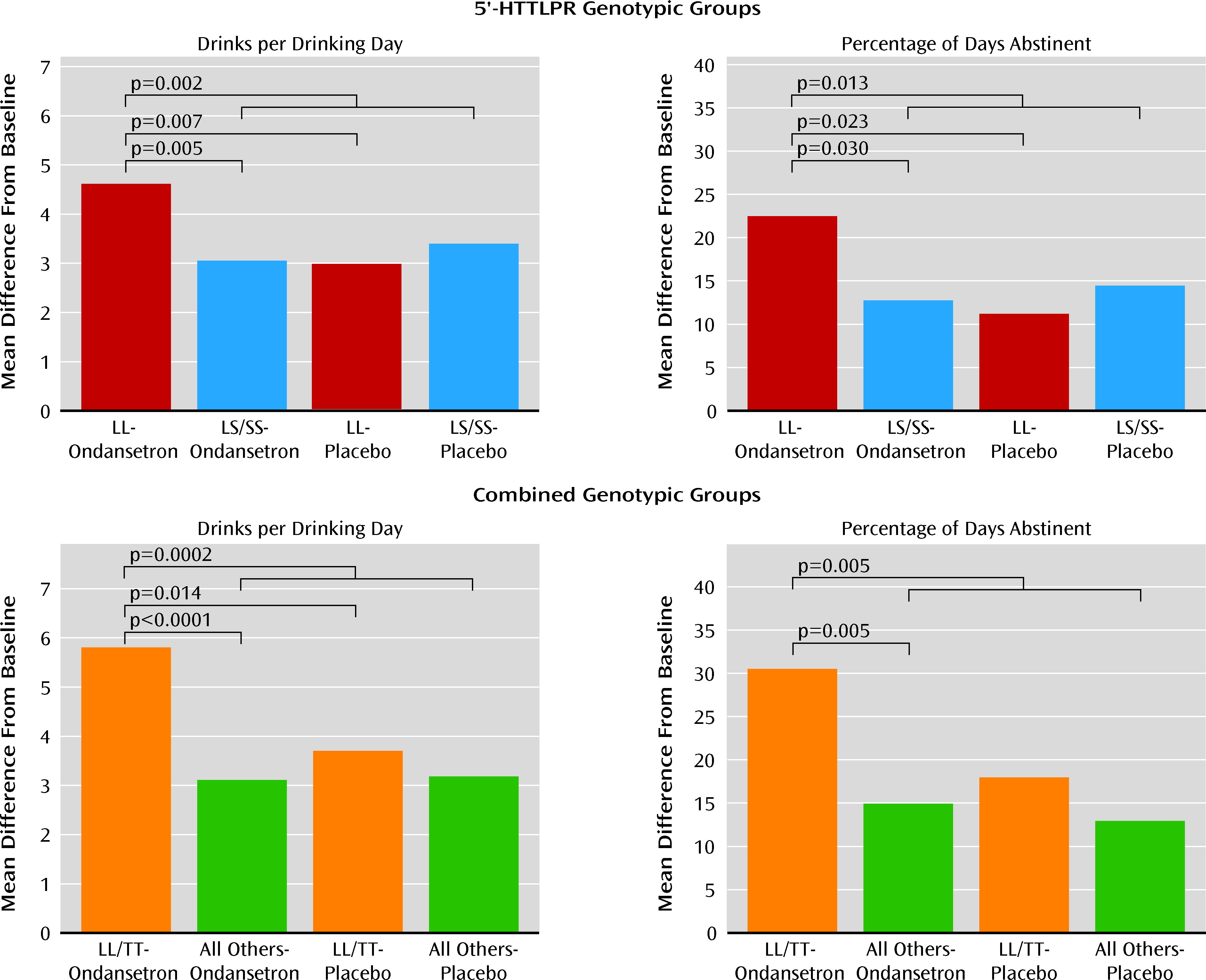
In our first model, for drinks per drinking day and percentage of days abstinent—two drinking measures with a statistically significant (p<0.05) correlation coefficient of −0.35—there was a significant interaction between 5′-HTTLPR genotype and treatment (F=6.68, p=0.010, and F=4.33, p=0.038, respectively). In the ondansetron group, LL compared with LS/SS participants had a significantly lower number of drinks per drinking day (mean difference=−1.53; 95% confidence interval [CI]=−2.59 to −0.47, p=0.005; effect size=0.47) and a significantly greater percentage of days abstinent (mean difference=9.73; 95% CI=0.95 to 18.50, p=0.03; effect size=0.29) (
Figure 2). In individuals with the LL genotype, the ondansetron group compared with the placebo group had a significantly lower number of drinks per drinking day (mean difference=−1.62; 95% CI=−2.79 to −0.46, p=0.007; effect size=0.56) and a significantly greater percentage of days abstinent (mean difference=11.27; 95% CI=1.55 to 21.00, p=0.023; effect size=0.41) (
Figure 2). Also, the ondansetron LL group compared with all the other genotype and treatment groups combined had a significantly lower number of drinks per drinking day (mean difference=−1.45; 95% CI=−2.37 to −0.54, p=0.002; effect size=0.45) and a significantly greater percentage of days abstinent (mean difference=9.65; 95% CI=2.05 to 17.25, p=0.013; effect size=0.32). For both outcome measures, significant results emerged in the third week of the study.
There was a significant interaction between the rs1042173 and 5′-HTTLPR genotypes for both drinks per drinking day (F=5.21, p=0.023) and percentage of days abstinent (F=6.85, p=0.009). Using the likelihood ratio test, the p value for adding three additional terms associated with rs1042173 genotype in the model was 0.025 for drinks per drinking day and 0.019 for percentage of days abstinent. Hence, the rs1042173 genotype added to the effect of 5′-HTTLPR genotype on both outcome measures. Also, we found that there was a significant main effect of the combined genotypes and treatment for both drinks per drinking day (F=4.14, p=0.001) and percentage of days abstinent (F=2.61, p=0.023). The largest reduction in drinks per drinking day (mean difference=−2.67; 95% CI=−4.01 to −1.33, p<0.0001; effect size=0.86) and increase in percentage of days abstinent (mean difference=17.98; 95% CI=6.83 to 29.14, p=0.002; effect size=0.65) were seen in the comparison of ondansetron recipients with the LL/TT genotype with all participants who received placebo. Notably, however, in the ondansetron LL/TT group compared with all others who received ondansetron, there was a significant reduction in number of drinks per drinking day (mean difference=−2.57; 95% CI=−3.93 to −1.21, p=0.0002; effect size=0.92) and a significant increase in percentage of days abstinent (mean difference=15.50; 95% CI=4.14 to 26.86, p=0.008; effect size=0.62). The difference in drinks per drinking day between LL/TT ondansetron and LL/TT placebo recipients was also significant (mean difference=−2.06; 95% CI=−3.72 to −0.39, p=0.015; effect size=0.72). Perhaps because of the relatively small sample size for a dichotomous variable, the difference in percentage of days abstinent between the LL/TT ondansetron and LL/TT placebo recipients was marginal but not significant (mean difference=12.52; 95% CI=−1.43 to 26.47, p=0.079; effect size=0.51).
Notably, the effect of ondansetron in reducing the number of drinks per drinking day and increasing the percentage of days abstinent was also greater in those with the LL/TT genotype compared with LL/G carriers (number of drinks per drinking day, mean difference=−2.34; 95% CI= −3.98 to −0.71, p=0.005; effect size=0.82; percentage of days abstinent, mean difference=15.25; 95% CI=1.51 to 28.98, p=0.03; effect size=0.62). The concomitant possession of the TT genotype appears to increase treatment response among those with the LL genotype.
The therapeutic effect sizes for LL and the LL/TT combination for ondansetron, compared with all the other ge-notype and treatment combinations, were 0.45 and 0.89, respectively, for drinks per drinking day, and 0.32 and 0.63, respectively, for percentage of days abstinent. (Effect sizes of 0.2, 0.5, and 0.8 are small, medium, and large, respectively [39].)
There was no significant difference in the overall drug use rate between any of the treatment groups, and the overall rate was 58.0%. The three most commonly used drugs were nicotine, cannabis, and cocaine (53.0%, 17.7%, and 4.9%, respectively).
For all participants, we observed a mean pill-taking rate of 62.7%, a mean breath alcohol concentration below the lowest detectable value of 0.01%, and a mean alcohol withdrawal score of 0.86 on the revised Clinical Institute Withdrawal Assessment for Alcohol scale. There were no significant group differences on these parameters.
No untoward medical consequences occurred that resulted in death, were life-threatening, required inpatient hospitalization, or resulted in persistent or significant disability or incapacity. Also, no adverse events occurred with significantly greater frequency for ondansetron recipients than for placebo recipients, or vice versa (all p values >0.05), except fatigue (p=0.019). The percentages of individuals in the ondansetron and placebo groups with at least one occurrence of the five most commonly reported adverse events were as follows: insomnia (20.5% and 22.3%, respectively), headache (20.9% and 19.4%, respectively), appetite disturbance (18.0% and 20.1%, respectively), fatigue (18.0% and 11.7%, respectively), and diarrhea (13.1% and 15.2%, respectively).
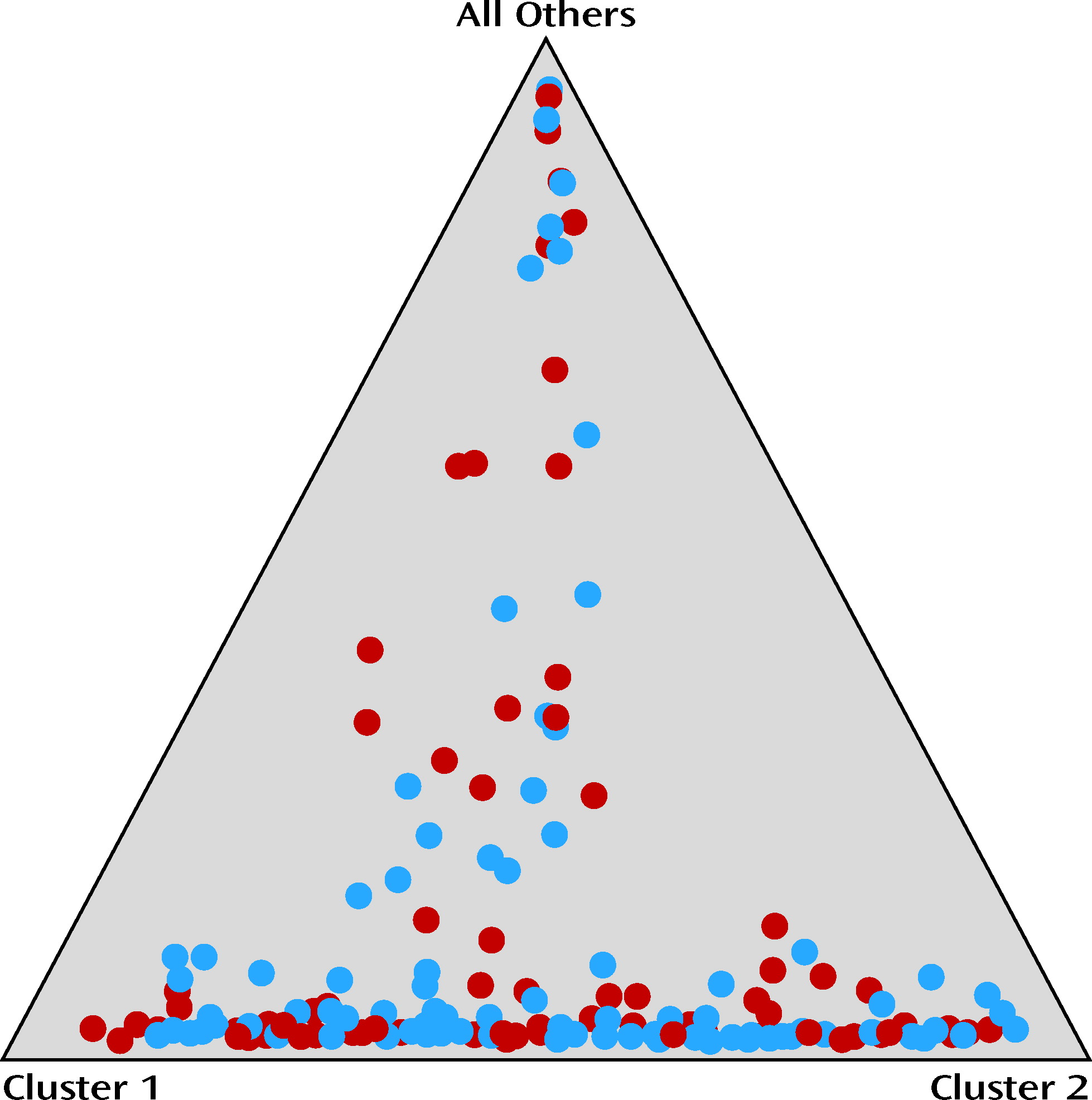
Population stratification for all participants included in the intent-to-treat sample, using the 24 ancestry-informative SNP markers, showed that there was no significant difference in population structure between the ondansetron and placebo groups. The range of average proportions for the two clusters (i.e., genetic ancestry) was 0.15–0.43 in the ondansetron group and 0.18–0.40 in the placebo group, and the mean value of alpha was 0.091 (
Figure 3).
Discussion
Our findings show that ondansetron is a promising therapeutic agent for the treatment of severe drinking among alcohol-dependent individuals with the LL genotype of the 5′-HTTLPR. These findings are supported by a pilot human laboratory study in which ondansetron suppressed alcohol self-administration significantly more in the LL compared with the LS/SS genotype among non-treatment-seeking alcoholics (
47).
From a qualitative clinical perspective, the relationship between drinking level and health consequences is not linear. For men, who constituted 73% of our sample, a high-risk level of drinking (five or more drinks per drinking day) is associated with severe health consequences, including accidental injuries (
48), death from external sources (
49), and being a target of aggression or committing an aggressive act (
50), as well as numerous medical, legal, and occupational problems (
51). Therefore, using that clinical criterion, participants with the LL or LL/TT genotype treated with placebo remained in the high-risk drinking category, while those with the LL or LL/TT genotype treated with ondansetron were, on average, moved out of the high-risk drinking category. Thus, participants with the LL or LT/TT genotype who received ondansetron, compared with their counterparts who got placebo, had a qualitatively greater clinical improvement. Furthermore, the clinical importance of our findings was underscored by demonstrating that both the LL and the combination of LL/TT genotypes also were significant predictors of an increase in the percentage of days abstinent for those who received ondansetron compared with those who received placebo.
Interestingly, the concomitant possession of the TT genotype of rs1042173 among those with the LL genotype of the 5′-HTTLPR enhanced the treatment response to ondansetron. Obviously, the magnitude of this increased effect cannot be determined directly within the same individual because the LL and TT genotypes are located within the same 5-HTT gene. It can, however, be inferred indirectly from the finding that the treatment effect of ondansetron among those with the combined LL and TT genotypes is significantly greater than that of an undifferentiated group with the LL genotype with different allelic frequencies of the TT, TG, or GG genotype, and the combined LL/TT group that received ondansetron had the best outcome. Nevertheless, despite our finding of a statistical interaction between the LL and TT genotypes, the molecular mechanism for this added therapeutic effect remains to be determined.
The operative issue in the clinical practice of pharmacogenetics is to identify and treat patients who will respond best to a particular medication. Hence, our findings promulgate the clinical approach of identifying alcohol-dependent individuals who are likely to respond to ondansetron based on their genotype analysis at the 5-HTT gene (i.e., LLs with or without the TT genotype) and providing them with the medication.
Our primary outcome variable tested a specific hypothesis related to ondansetron's effects on the severity of drinking in alcohol-dependent individuals who varied by 5-HTT genotype. This specific pharmacogenetic hypothesis was proposed before the present study was conducted (
4) and was developed through systematic experimental studies (
3,
17). Testing a specific hypothesis is a conservative scientific approach because it avoids the presumption that a particular biological or genetic effect can be extrapolated to explain different patterns of drinking behavior. Consistent with this approach, we restricted the test of the effect of ondansetron response to just one other secondary variable—percentage of days abstinent—to provide additional information to clinicians. Our findings underscore the clinical importance of our results because they demonstrate that both LL and the combination of LL/TT genotypes predicted therapeutic response to ondansetron by increasing the percentage of days abstinent relative to placebo.
Notably, even though we enrolled into this clinical trial currently drinking alcohol-dependent individuals who had to have a breath alcohol concentration no greater than 0.02% for clinical attendance, the alcohol concentration levels reported in the trial were extremely low, and no significant withdrawal symptoms were reported. This phenomenon shows that alcohol-dependent individuals are able to moderate or taper their drinking levels, perhaps as a result of the behavioral contingency of monitoring drinking levels (
52). Also, this finding demonstrates the feasibility of providing treatment to alcohol-dependent individuals at the typical point of maximum crisis—when they are actively drinking alcohol and asking for help—and suggests that prior detoxification is not always a prerequisite for the treatment of alcohol-dependent individuals who can be managed in an outpatient setting.
Our study was limited by five factors. First, we did not have equal numbers of individuals from the different ethnicities and hence could not test formally for a specific effect of ethnicity on genetic profile; however, the general trend and magnitude of the differences reported were similar for whites and Hispanics (data not shown). Additionally, we performed an analysis for genetic structure with 24 unlinked ancestry-informative markers—which has been reported to be as effective in estimating continental population ancestry as analyses with more than 90 ancestry-informative markers (
38)—and these ancestry proportion estimates were used as covariates in all our statistical models in place of self-defined ethnicity. The ancestry proportions, estimated in each of the three parental population groups, were symmetric between samples in the ondansetron and placebo groups (data not shown). Furthermore, we did not find in our statistical analyses that ancestry proportions were a significant covariate. Second, not all those who received ondansetron were treated successfully—only those with a specific allelic constitution of the 5-HTT gene. Hence, more research is needed to find alcoholics with other genetic polymorphisms who will respond significantly to alternative medications. Third, there might be other genetic variants within the 5-HTT gene that could increase further the specificity of ondansetron's treatment response; such relationships can be elucidated only through larger-scale pharmacogenomic studies. Fourth, the molecular mechanisms associated with the differential effect in the severity of drinking on L and S variants of the 5′-HTTLPR and T and G alleles of the 3′-UTR are not well understood; thus, additional in vitro and in vivo studies are needed. Fifth, while we considered the possibility that comorbid depression or anxiety-related disorders that have shown an association with serotonergic genotypes could confound our findings, we think this unlikely since this cohort excluded such individuals.
We minimized type I error by performing inferential analysis only to test prespecified pharmacogenetic hypotheses on a single primary endpoint and by doing only pairwise comparisons within different genotype groups when there was a significant interaction effect between treatment and genotype.
Given our earlier finding (
28) that early-onset alcoholics were more likely than late-onset alcoholics to respond therapeutically to ondansetron treatment, we considered the possibility that age at onset could be a proxy for serotonin transporter genotype, or vice versa. Early-onset alcoholics differ from late-onset alcoholics by having an earlier age at onset of alcohol-related problems, typically before age 25 (although this can vary by definition), and greater rates of impulse control disorders (
53). Notably, in the present cohort, there was no significant association between serotonin transporter genotype and age at onset (data not shown), and those with the LL or LL/TT genotype responded to ondansetron irrespective of age at onset. Despite the lack of a significant association between age at onset and genotype, and contrary to expectation (
54), the slightly stronger trend was for those who had the LL genotype and were late-onset alcoholics to respond better to ondansetron than the LL early-onset alcoholics (data not shown). This also was unexpected because the preponderance of the literature suggests that the S allele is more likely to be associated with an early onset of alcoholism (
55). As exemplified by the contradictory reports in the literature, age at onset is difficult to standardize, especially across different populations and ethnicities, and the debate as to the constellation and stability of alcohol-related problems that should be used to make this characterization extends back over 150 years (
53). In contrast, the measurement of genetic variation is extremely accurate and can be used reliably over time and across populations. A pharmacogenetic approach might therefore be a practical and useful method in general practice to identify those who should or should not receive ondansetron for the treatment of alcohol dependence.
In sum, the results of our study have enabled us to integrate a unique scientific hypothesis, and a systematic series of basic research and human laboratory studies, with new clinical knowledge to demonstrate the promise of a novel pharmacogenetic approach to reduce the severity of -alcohol consumption and increase abstinence among alcoholics with specific polymorphisms of the 5-HTT gene.
Acknowledgments
The authors thank the National Institute on Alcohol Abuse and Alcoholism for its generous support through grants 7 U10 AA011776-10, 7 R01 AA010522-12, 2 R01 AA010522-13, 5 R01 AA013964-05, 5 R01 AA014628-04, and 5 R01 AA012964-06 to Dr. Johnson, grant 5 K23 AA000329-06 to Dr. Ait-Daoud, and grant RC1 AA019274 to Dr. Liu; the National Institute on Drug Abuse for its support of Dr. Li through grants DA012844 and DA013783; and Robert H. Cormier, Jr., B.A., and Ann Richards, B.A., for their assistance with manuscript preparation.