Bipolar disorder affects 1.5%–3% of the population (
1), yet the neurophysiologic basis for the disorder remains unknown. Structural neuroimaging studies using region-of-interest-based techniques have reported that bipolar patients exhibit volumetric reductions in gray matter subregions of the frontal lobe, including the left subgenual (
2,
3), the left dorsomedial (
4), the left and right inferior and middle prefrontal (
4,
5), and the left and right anterior cingulate cortices (
6). However, other region-of-interest-based studies of bipolar disorder have reported greater volumes (
7) or no difference (
8) in these regions relative to healthy volunteers. Studies using methods that compare gray matter on a point-by-point basis, such as voxel-based morphometry, have found similar gray matter deficits in the left subgenual (
9), the left middle (
9) and the left and right inferior prefrontal (
10), and the left anterior cingulate cortices (
10). Yet other voxel-based morphometry studies have found either no difference in frontal regions (
11,
12) or a greater volume of gray matter in patients relative to healthy volunteers (
13).
Discussion
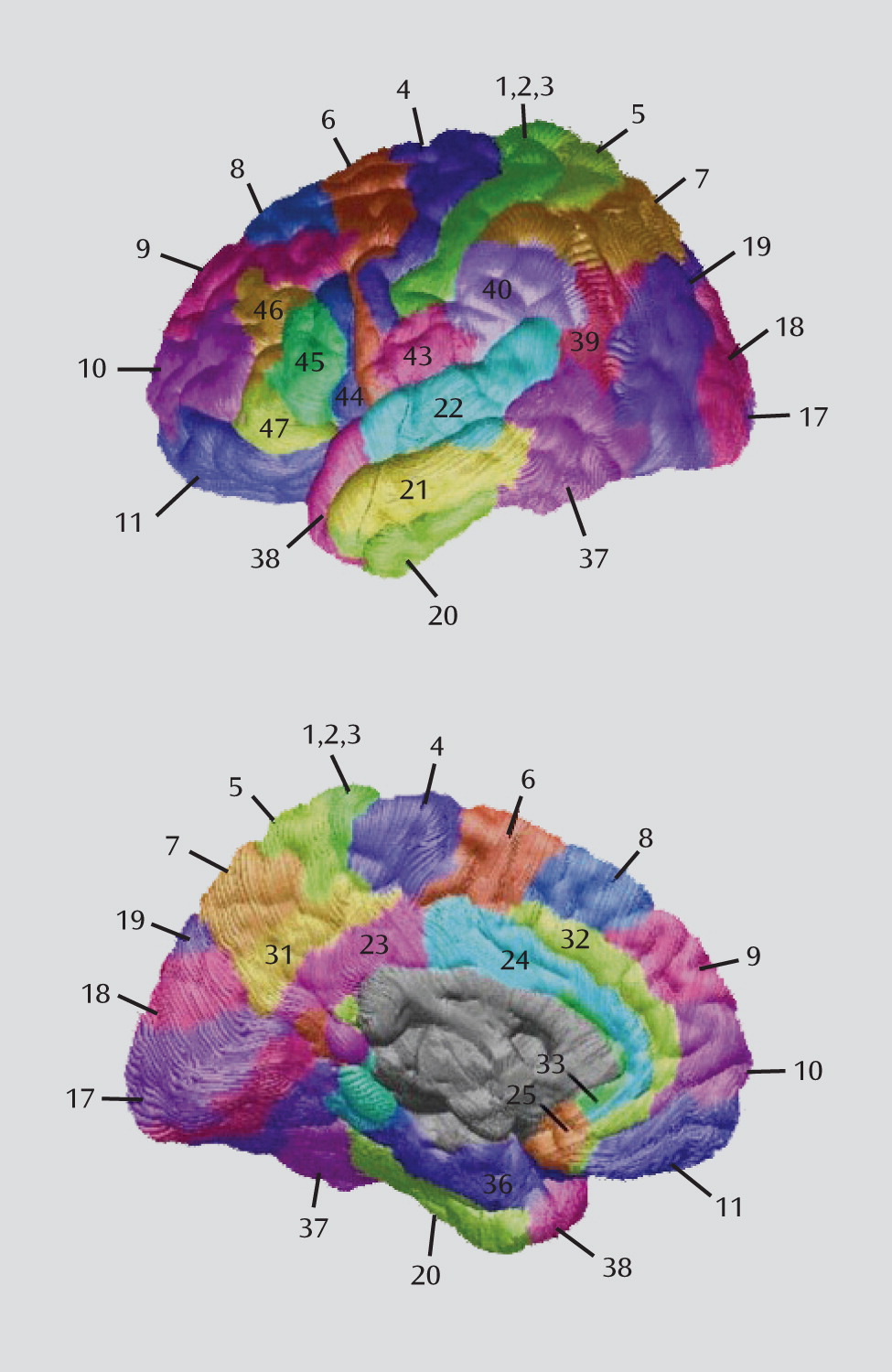
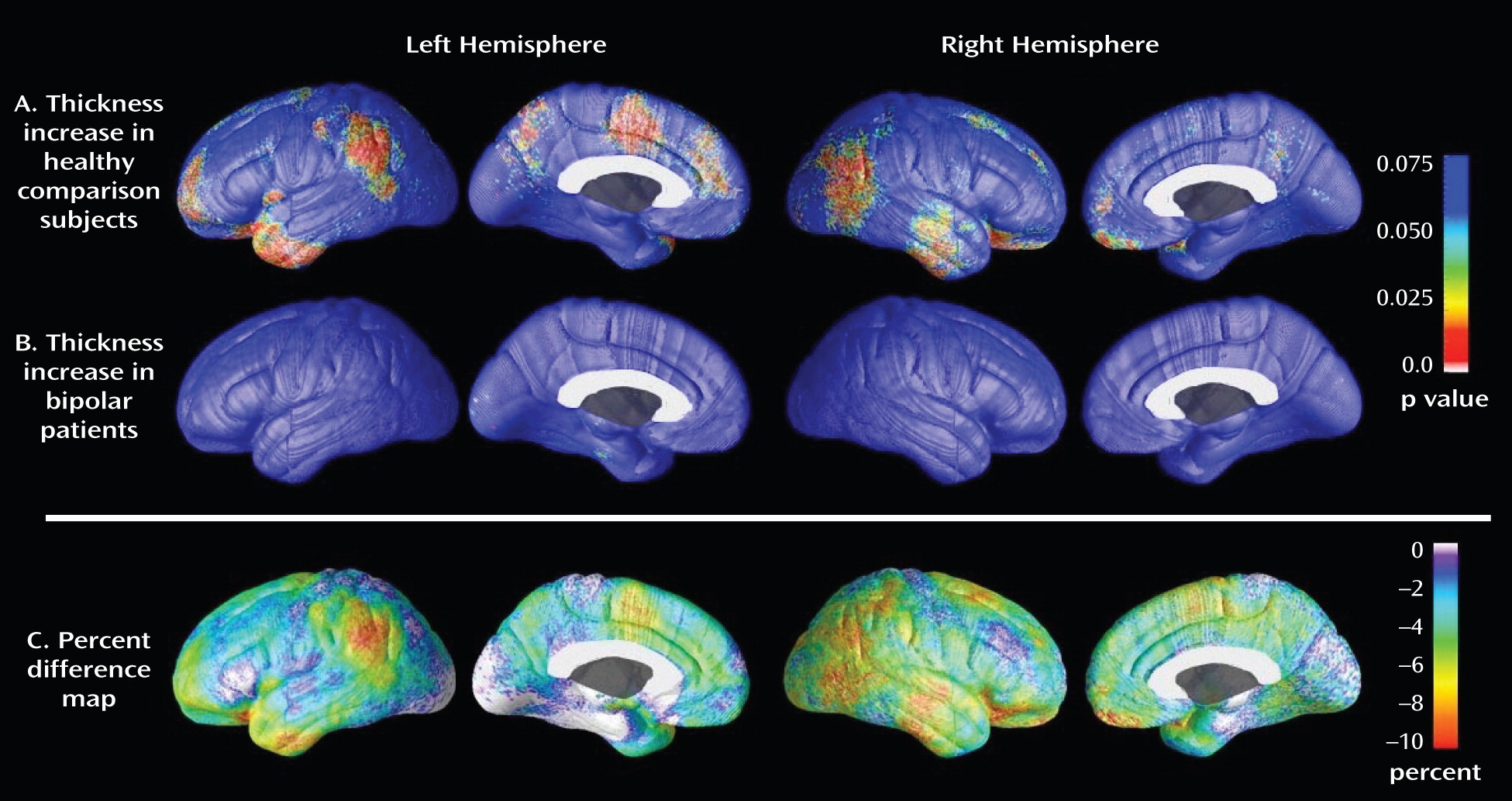
Using cortical matching methods in conjunction with tools for measuring gray matter thickness, we found significant thinning in the left and right prefrontal and the left anterior cingulate cortices in euthymic bipolar patients relative to healthy comparison subjects. Within these regions, thinning was localized to specific subregions, including the left and right orbital cortex (Brodmann's area 11), the left frontopolar cortex (Brodmann's area 10), the left dorsomedial cortex (Brodmann's area 8), the left ventrolateral prefrontal cortex (Brodmann's area 44), the left anterior cingulate cortex (Brodmann's area 24), and the left pericingulate cortex (Brodmann's area 32).
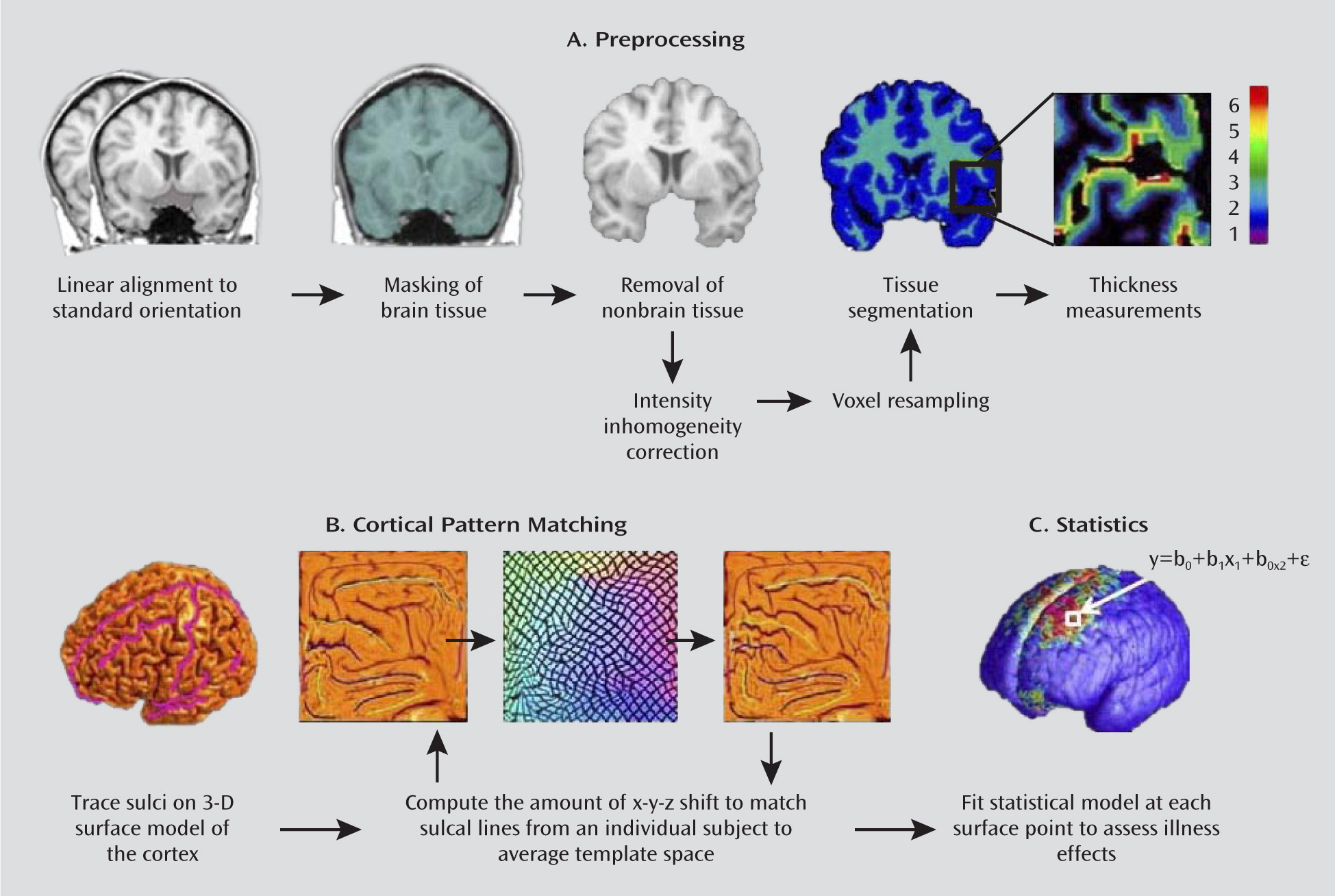
To our knowledge, only two previous studies have examined brain structure in recurrently ill adult patients with bipolar disorder using cortical thickness methods in conjunction with MRI. Lyoo et al. (
29) reported thinning of prefrontal cortical gray matter in 25 bipolar patients relative to 21 healthy comparison subjects in left Brodmann's areas 46, 24, and 32 and right Brodmann's area 10. In a region-of-interest-driven study, Fornito et al. (
6) found thinning in left Brodmann's area 24 and right Brodmann's area 32 in 24 patients relative to 24 healthy comparison subjects. In the present study, which used a larger sample of 34 patients and 31 healthy comparison subjects, cortical pattern matching methods were used to allow a more precise mapping of thickness abnormalities in bipolar disorder (
30,
31). These methods improve on the traditional registration approaches by using sulcal features to align corresponding anatomy across participants, eliminating much of the confounding anatomical variance when pooling data across participants, thereby making it easier to identify and localize subtle group differences in brain structure (
19). Using this highly sensitive technique, we replicated and extended the above prior study findings.
This study has several unique strengths. First, unlike a majority of previous studies, which either did not specify current medication treatment (
32) or included a number of patients who were receiving treatment with lithium at the time of scanning (
4,
8,
10,
12,
29), all participants in our patient sample were free of current treatment with this medication. This aspect of the study may be particularly important given recent evidence showing that lithium treatment is associated with significant increases in cortical gray matter. Moore et al. (
33) found that total gray matter volume increased by 3%, on average, in bipolar patients after 4 weeks of lithium treatment. Sassi et al. (
16,
34) found larger total gray matter volume in lithium-treated bipolar patients compared with both untreated patients and healthy comparison subjects. Bearden et al. (
14) found that prefrontal cortical gray matter density was greater in bipolar patients treated with lithium relative to both healthy comparison subjects and bipolar patients not treated with lithium. Our group has also found lithium-associated gray matter enlargement of subcortical structures in bipolar patients (
15). Use of lithium, therefore, may serve to explain why some previous structural neuroimaging studies of bipolar disorder have either failed to detect gray matter reductions in patients (
11) or found group differences in the opposite direction (i.e., larger gray matter volumes in bipolar patients relative to healthy comparison subjects) (
7,
12,
13).
A second strength of this study is that all participants in our bipolar group were in the same mood state (euthymia) at the time of scanning. Although previous studies have not controlled for this factor, recent data from our group (
17) and others (
8) suggest that mood state may affect MRI results. Brooks et al. (
17) found that depressed bipolar patients exhibited lower gray matter density in the dorsal prefrontal cortices relative to euthymic bipolar patients. Nery et al. (
8) also found gray matter reductions in the orbitofrontal cortex of depressed relative to euthymic bipolar patients. A similar pattern of gray matter volume reductions was found more recently by our group in patients scanned longitudinally (in different mood states) as well (
35a).
A third strength of our study is that all participants in the patient group had diagnoses of bipolar I disorder. Whether bipolar subtype is associated with distinct cortical abnormalities is not known, but subtype could contribute to the heterogeneity of structural neuroimaging findings. Only one study (
35), to our knowledge, has specifically examined the impact of bipolar subtype on brain structure. In that study, unmedicated patients with bipolar I disorder were found to exhibit smaller volumes of the left amygdala compared with unmedicated patients with bipolar II disorder. This finding, as well as data from studies that show bipolar I disorder to be associated with greater neuropsychological impairment (
36), a higher risk for psychosis, and more severe manias than bipolar II disorder, suggests that bipolar subtype could be associated with distinct patterns of thinning in cortical gray matter. Studies that directly compare cortical gray matter in patients with bipolar I and bipolar II disorder are needed to more thoroughly examine this issue.
With these efforts to study a more homogeneous bipolar population and to control for some known confounders, we found reduced thickness in the prefrontal and anterior cingulate cortices of patients with bipolar disorder. The etiology of this thinning remains to be elucidated. One possibility is that reduced thickness in the prefrontal and anterior cingulate cortex of bipolar patients is the result of an underlying neurodegenerative process associated with possible toxic effects of mood episodes. In line with this, and consistent with previous studies (
28,
29,
37), we found significant widespread negative associations between cortical thickness and previous course of illness. Although it is tempting to speculate that decreased cortical thickness in patients is causally associated with greater illness duration and a higher number of previous depressive episodes, because these variables were highly correlated with age, we could not disentangle these effects. Studies that sample from bipolar patient groups with a narrow age range but a wide range in the number of previous depressive episodes or other course-of-illness measures are needed to tease apart these factors.
Another possibility is that reduced cortical thickness is present early in the course of the disorder, possibly prior to illness onset, and that such thinning alters normal inhibitory cortico-limbic networks, resulting in an increased vulnerability to emotion dysregulation. Data to support this hypothesis come from studies finding gray matter abnormalities in the prefrontal cortex of unaffected relatives of individuals with bipolar and unipolar depressive disorders (
38,
39). Whether these individuals with cortical thinning go on to develop the disorder is unclear, however, and longitudinal studies that more thoroughly explore the relation between cortical thinning and illness onset would be of interest.
Thinning of the prefrontal and anterior cingulate cortices could contribute to some of the behavioral changes that are observed in bipolar disorder. Lesion studies show that focal damage to the orbitofrontal cortex is associated with a diminished ability to properly gauge the positive or negative emotional consequences of one's actions (
40), and damage to the anterior cingulate cortex results in symptoms that include inattention and emotional instability (
41,
42). At least one functional neuroimaging study (
43) has reported activation in both the orbitofrontal and anterior cingulate cortices in healthy volunteers during the conscious regulation of negative emotional states. This same study found that activation in the orbitofrontal cortex was negatively correlated with activation in the amygdala, suggesting an inhibitory connection between these regions. Other studies involving emotion regulatory paradigms, however, have found frontal activation (and corresponding negative correlations with amygdala activation) to be localized to the lateral portion of prefrontal cortex (e.g., Brodmann's area 47) (
44–
46). Although thinning in this ventrolateral region was not observed in patients in the present study, it is well established that robust structural connections run between the lateral (e.g., Brodmann's area 47) and medial (e.g., Brodmann's area 11) sectors of the orbitofrontal cortex, but that only the medial subdivision sends direct inhibitory projections to the amygdala (
47,
48). The lateral sector of the orbitofrontal cortex therefore may suppress amygdala output via intermediary projections from the medial orbitofrontal cortex. Thus, it is interesting that our group has observed increased activation of the amygdala and decreased activation of the lateral orbitofrontal cortex (
44,
49,
50) and thinning in the medial (but not lateral) orbitofrontal cortex. The data in this study could therefore provide a structural etiology for the functional abnormalities previously observed by our group. Additional studies examining the relation between brain structure and function in emotion regulatory circuits are currently under way in our laboratory.
With the exception of thinning in the orbitofrontal cortex, which was bilateral, most of the thinning observed in patients in the present study was localized to the left hemisphere. This hemispheric pattern agrees with previous studies; of the four that have reported abnormal structure of the anterior cingulate (i.e., Brodmann's area 24/32), three (
10,
29,
51) found deficits in the left hemisphere only, one (
6) found deficits in the anterior cingulate bilaterally, and none found deficits that were restricted to the right hemisphere. The reason for this laterality is not known, although the concept of hemispheric lateralization of mood regulation is well documented. Current models of emotional processing suggest that positive (or approach-related) emotions are lateralized toward the left hemisphere, whereas negative (or withdrawal-related) emotions are lateralized toward the right hemisphere (
52). Lesions to the left prefrontal cortex, for example, have been associated with an increased risk for depressive symptoms (
53).
In addition to the negative associations observed here between cortical thickness and illness duration and number of previous depressive episodes, two other findings require further investigation. First, thickness in the left subgenual prefrontal cortex was positively associated with the number of past hospitalizations for mania, a finding that remained significant when age was controlled for in our statistical model. It is possible, given recent evidence suggesting that mood state may affect brain structure (
8,
17), that the manic state itself, particularly when severe enough to require hospitalization, has enduring hypertrophic effects on gray matter. Additional research, however, is needed to address this possibility. Second, a history of psychosis in bipolar patients was associated with significantly greater thinning in the left ventrolateral prefrontal cortex (Brodmann's area 44), the left dorsomedial prefrontal cortex (Brodmann's area 8), and the left temporal pole (Brodmann's area 38). More pronounced thinning in these regions may suggest that psychotic and nonpsychotic forms of bipolar disorder are characterized by distinct patterns of gray matter abnormalities. This pattern of deficit is congruent with neurocognitive studies that show bipolar patients with a history of psychosis to be impaired on some prefrontal functions, such as executive functioning and spatial working memory, compared with bipolar patients without such a history (
54). Moreover, studies of patients with chronic schizophrenia (
55,
56) and psychosis (
57,
58) consistently show gray matter reductions in the left dorsomedial region of the prefrontal cortex (Brodmann's area 8). Cortical gray matter loss in this region therefore may be associated with psychosis in particular. In the bipolar patients in the present study, however, unlike in patients with schizophrenia, cortical gray matter in the dorsolateral prefrontal cortex (e.g., Brodmann's area 46) was relatively spared. Thus, distinct and identifiable patterns of neuroanatomical pathology could potentially distinguish these two disorders. However, additional studies are needed to more accurately address this possibility.
Our study has several limitations. First, many of our patients were taking other medications, of which the long- and short-term effects on brain structure are not known. Our comparisons of cortical thickness between medicated and unmedicated patients, however, showed no evidence of a significant effect of this factor. Second, some patients reported taking lithium in the years prior to scanning, and past exposure to this medication could have affected our results. As lithium increases gray matter volume (
33), however, it would be expected that any enduring hypertrophic effects of past treatment with this medication would have produced group differences opposite to those observed here. Additionally, comparisons between patients who had and had not previously taken lithium indicate that prior lithium use itself was not a significant confounder.