Cognitive deficits, which represent a treatment-resistant, disabling symptom domain in schizophrenia, are attributable, at least in part, to alterations in cortical interneurons that utilize GABA as an inhibitory neurotransmitter (
1–
4). These alterations appear to be prominent in the subset of GABA neurons that express parvalbumin. For example, in the prefrontal cortex of subjects with schizophrenia, parvalbumin neurons exhibit lower mRNA and protein levels of GAD67 (the 67-kDa isoform of glutamic acid decarboxylase, an enzyme for GABA synthesis) (
5,
6), reduced immunoreactivity of GABA transporter 1 (a presynaptic GABA transporter) (
7), and lower parvalbumin mRNA expression (
5,
8–
10). Because parvalbumin neurons are essential for the generation of γ-oscillations that appear to be a neural substrate for cognitive functions (
11,
12), the alterations in parvalbumin neurons are thought to contribute to γ-oscillation disturbances and cognitive deficits in schizophrenia (
3,
13).
We recently demonstrated (
14) the selective expression of KCNS3, the gene encoding Kv9.3 voltage-gated potassium channel modulatory α-subunit, in parvalbumin neurons in the human prefrontal cortex. In heterologous expression systems, Kv9.3 subunits do not assemble into homomeric channels but form functional heteromeric channels with delayed rectifier Kv2.1 α-subunits (
15–
17), which are expressed by the majority of cortical neurons, including parvalbumin neurons (
18). Compared with homomeric Kv2.1 channels, heteromeric Kv2.1/Kv9.3 channels have faster activation, slower deactivation and inactivation, and steady-state activation and inactivation curves that are shifted toward more negative values by ∼20 mV (
15,
16). Therefore, Kv2.1/Kv9.3 channels appear to be more effectively activated by subthreshold membrane depolarizations such as those generated by excitatory synaptic inputs.
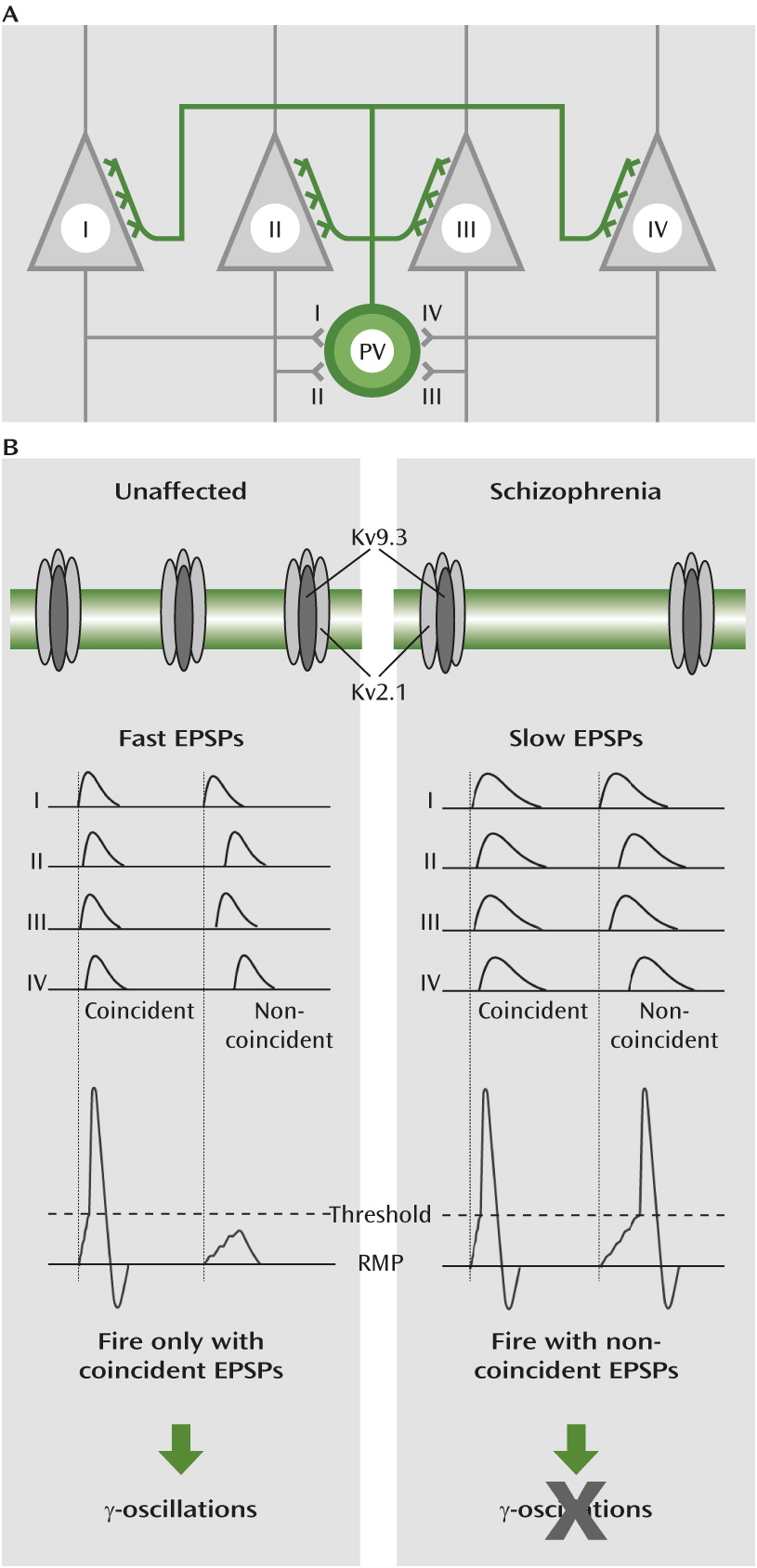
Parvalbumin neurons have a faster decay of excitatory postsynaptic potentials than other types of cortical neurons (
19–
21). Because fast potentials summate within a short time window (
22), parvalbumin neurons fire action potentials only if they receive highly coincident excitatory inputs. Activation of voltage-gated potassium channels during excitatory postsynaptic potentials is one mechanism for the fast potentials in parvalbumin neurons (
22). Therefore, the selective expression of KCNS3-encoded Kv9.3 subunits in parvalbumin neurons appears to contribute to the ability of parvalbumin neurons to detect coincident excitatory synaptic inputs that reflect synchronized cortical activities, and thus might contribute to their critical role in generating γ-oscillations.
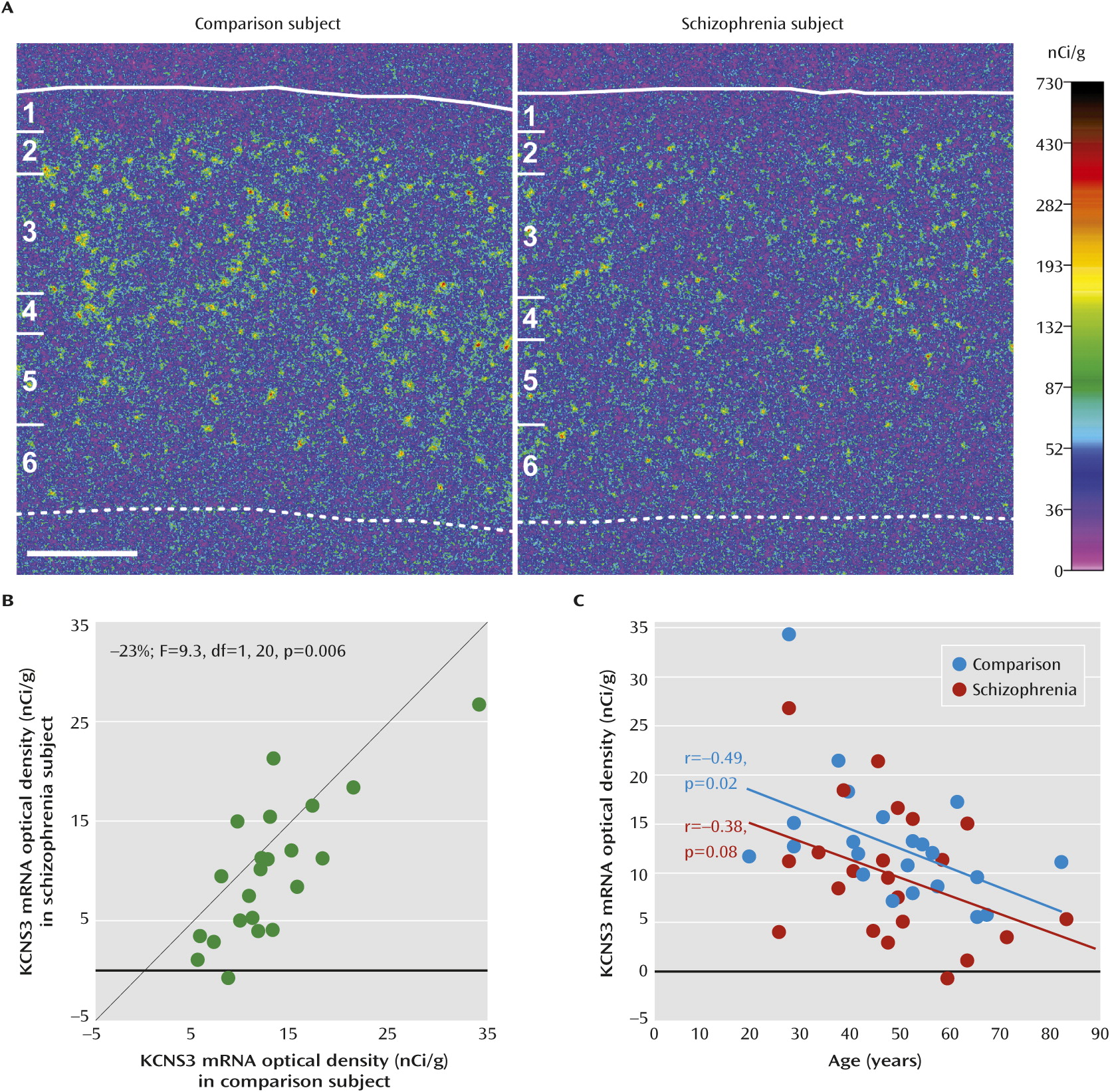
Given the evidence for impaired γ-oscillations in schizophrenia, we sought to determine whether KCNS3 expression is altered in the prefrontal cortex of subjects with schizophrenia. Two complementary approaches in separate cohorts of subjects were used. In one cohort, KCNS3 mRNA was visualized by in situ hybridization and quantified at both the tissue and cellular levels. In the second cohort, parvalbumin neurons were identified by the presence of perineuronal nets and captured by laser microdissection; KCNS3 and KCNB1 (which encodes Kv2.1) mRNAs were measured by microarray.
Discussion
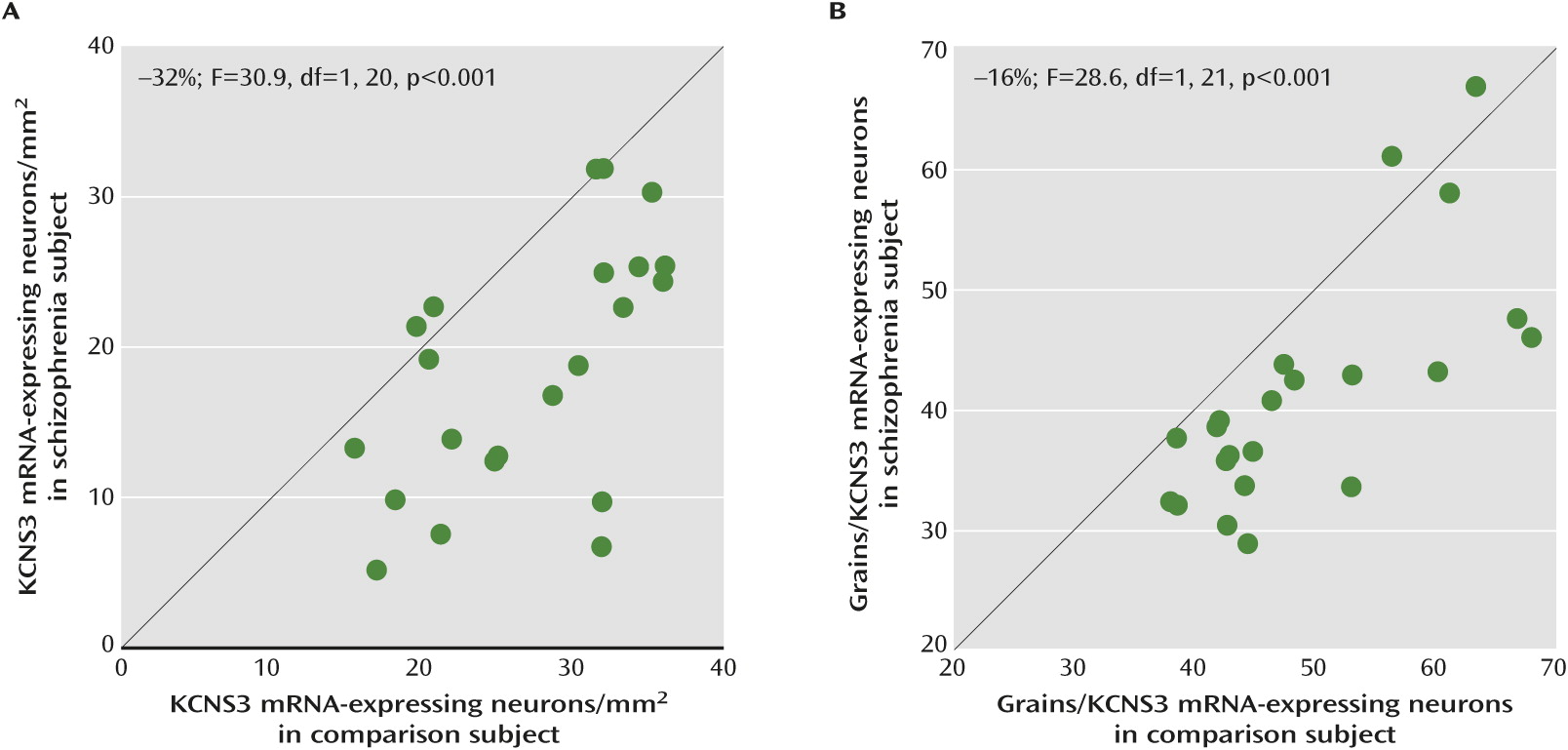
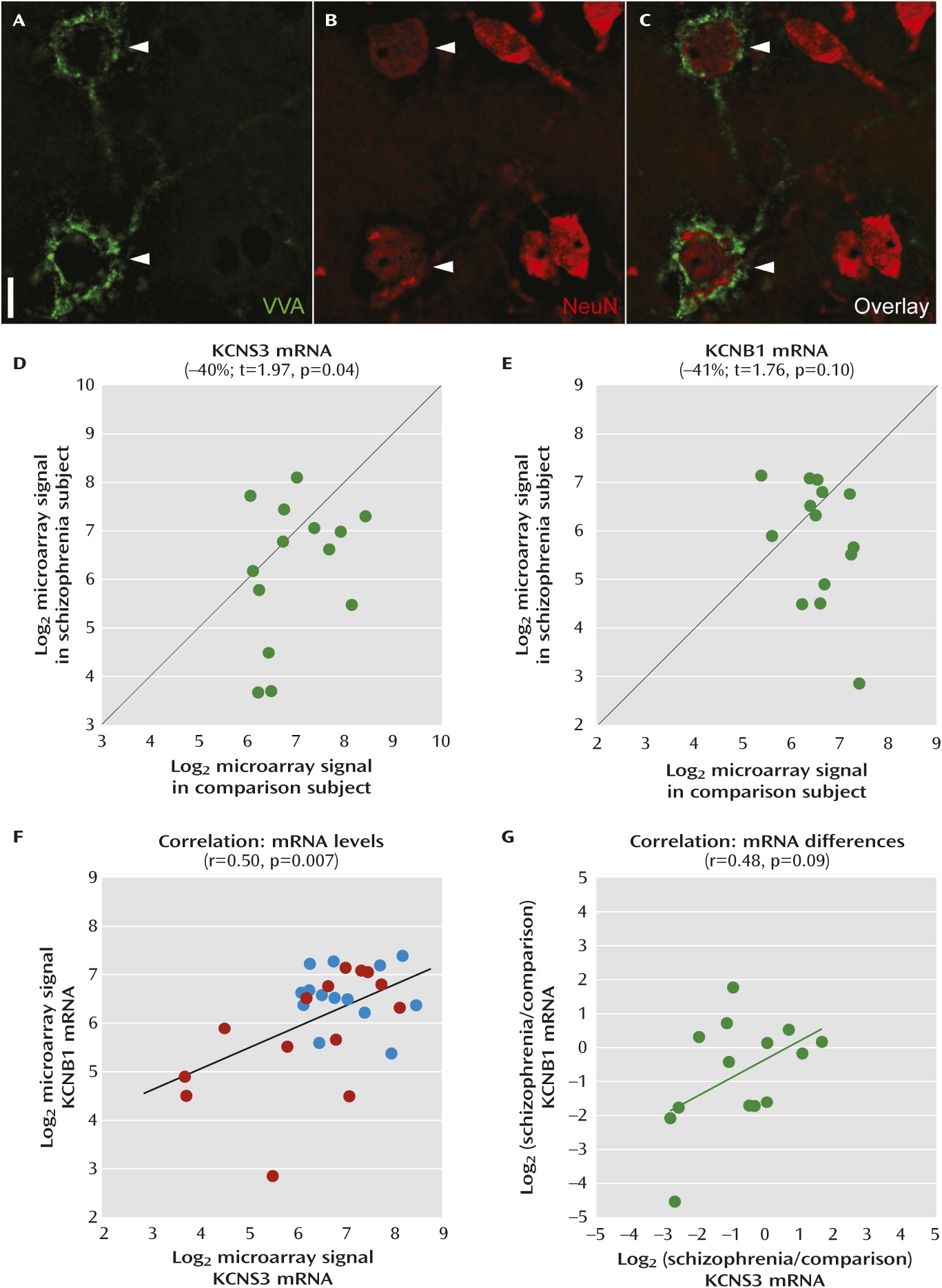
In schizophrenia, previous postmortem studies revealed various abnormalities in cortical GABA neurons, including expression deficits of genes that regulate GABA neurotransmission (
9,
23,
30). Some of these changes are prominent in parvalbumin neurons and are thought to underlie disturbances in γ-oscillations in schizophrenia (
3,
13). In this study, we focused on the parvalbumin neuron-selective KCNS3 voltage-gated potassium channel subunit gene that appears to have a physiological function in the generation of γ-oscillations by parvalbumin neurons. Our in situ hybridization analyses of 22 pairs of schizophrenia and matched comparison subjects revealed that the density of cortical KCNS3 mRNA-expressing neurons and the mean KCNS3 mRNA level per neuron were reduced in the schizophrenia subjects, indicating that KCNS3 mRNA levels are decreased in the majority of parvalbumin neurons and below the threshold of detection in a subset of severely affected parvalbumin neurons. Consistently, microarray analyses specifically of prefrontal cortex neurons labeled with VVA, a selective marker of parvalbumin neurons, in a separate cohort of 14 subject pairs detected significantly lower KCNS3 mRNA levels in schizophrenia subjects. In addition to previous findings in parvalbumin neurons, such as lower GAD67 expression, that appear to represent a molecular basis for reduced magnitude of GABA neurotransmission by these neurons (
3), the lower KCNS3 mRNA levels in prefrontal cortical parvalbumin neurons, demonstrated by two different methods in two nonoverlapping cohorts, could provide another molecular mechanism of parvalbumin neuron dysfunction that might also underlie the disturbances in γ-oscillations in schizophrenia.
Consistent with the reduced density of cortical perineuronal nets in schizophrenia (
31), VVA labeling appeared to be lower in the schizophrenia subjects. This observation raises the possibility that some perineuronal nets were not visible, and thus the corresponding parvalbumin neurons were not sampled in schizophrenia subjects. However, a reduction in VVA labeling in schizophrenia would likely bias against finding a difference in KCNS3 expression in schizophrenia, as the most affected cells would be the least likely to be captured and assayed.
Several lines of evidence indicate that the reduction in KCNS3 mRNA expression is due to the disease process of schizophrenia and not attributable to potential confounding factors frequently associated with the illness. First, among the 22 schizophrenia subjects analyzed by in situ hybridization, four subjects who were not taking antipsychotic medications at time of death demonstrated lower KCNS3 mRNA levels relative to their matched comparison subjects, similar to the lower levels seen in 18 schizophrenia subjects on medication at time of death (Figure S2). Second, KCNS3 mRNA expression was unaltered in the prefrontal cortex of monkeys chronically exposed to either haloperidol or olanzapine (Figure S3). Third, none of the potentially confounding clinical factors, such as suicide, socioeconomic status, substance abuse or dependence at time of death, and treatment with antidepressants or benzodiazepines/anticonvulsants at time of death, accounted for the lower KCNS3 mRNA expression in the gray matter or individual VVA-labeled neurons in schizophrenia subjects (Figure S2).
A significant negative correlation between age and the KCNS3 mRNA expression was observed in comparison subjects, and the correlation fell short of significance in schizophrenia subjects. The regression line for schizophrenia subjects was parallel to and shifted downward from that of comparison subjects (
Figure 1C), indicating that the decreased expression of KCNS3 mRNA in schizophrenia is present across adult life and thus unlikely to be a consequence of illness chronicity. Furthermore, this observation suggests that the KCNS3 expression deficit may be present early in the course of the illness and thus could contribute to the pathophysiology of the clinical features of the illness.
KCNS3 mRNA encodes potassium channel Kv9.3 modulatory α-subunits. Kv9.3 subunits assemble with ubiquitously expressed Kv2.1 α-subunits, with a 1:3 subunit stoichiometry, to form heteromeric Kv2.1/Kv9.3 channels (
17). Interestingly, in the microarray data of individually collected VVA-labeled neurons, KCNB1 mRNA, which encodes the Kv2.1 subunit, showed a decrease comparable to KCNS3 mRNA in schizophrenia (
Figure 3E), and expression levels of both transcripts tracked together (
Figure 3F,
3G). These findings suggest a concomitant down-regulation of Kv9.3 and Kv2.1 subunits, and thus a lower complement of Kv2.1/Kv9.3 heteromeric channels, in prefrontal cortical parvalbumin neurons in schizophrenia (
Figure 4).
The subcellular distribution of Kv2.1 immunoreactivity in cortical parvalbumin neurons supports the presence of Kv2.1/Kv9.3 heteromeric channels in the dendrites of individual parvalbumin neurons (
18). Compared with homomeric Kv2.1 channels, Kv2.1/Kv9.3 channels are more effectively activated by small depolarization steps from the resting membrane potential (
15,
16). These data suggest that Kv2.1/Kv9.3 channels are among those dendritic voltage-gated potassium channels that contribute to the acceleration of excitatory postsynaptic potential decay in parvalbumin neurons (
22). Because such fast potentials summate in a narrow time window (
22), parvalbumin neurons can efficiently detect temporally convergent excitatory inputs and fire action potentials (
Figure 4). The output of parvalbumin neurons simultaneously inhibits large groups of neighboring pyramidal neurons (
32,
33), leading to their synchronized firing when they concomitantly emerge from the hyperpolarized state (
34). This synchrony occurs as a γ-frequency oscillation because of the rate of decay of the pyramidal cell inhibition at synapses from parvalbumin neurons (
34). Because parvalbumin neurons receive excitatory inputs from neighboring pyramidal neurons (
35), the presence of Kv2.1/Kv9.3 channels in parvalbumin neurons enables them to fire action potentials in response to the coincident inputs from the synchronized activity of pyramidal neurons, sustaining γ-oscillations in the parvalbumin-pyramidal neuron network. Consequently, the deficient KCNS3 expression, and the concomitant down-regulation of KCNB1 expression in parvalbumin neurons, would be predicted to slow the time course of excitatory postsynaptic potentials in parvalbumin neurons, contributing to altered γ-oscillations (
Figure 4) and impaired cognitive function in schizophrenia (
13).
Abnormal cortical expression has been reported for other voltage-gated potassium channel subunit genes—namely, KCNC1 (Kv3.1) (
36) and KCNH2 (Kv11.1) (
37)—in schizophrenia, although the affected cell types were not determined. In contrast to the KCNS3-encoded Kv9.3 subunit that controls subthreshold membrane potentials (
15), these subunits regulate repolarization of action potentials (
37,
38), a process critical to the high-frequency and repetitive firing of parvalbumin neurons. Therefore, alterations in different types of potassium channel subunits may disturb different electrophysiological properties of parvalbumin neurons, which could converge on disturbances in γ-oscillations in schizophrenia. Because KCNS3 is selectively expressed in parvalbumin neurons, the identification of pharmacological compounds targeting Kv9.3 subunits may be useful for developing therapeutic strategies that selectively restore the function of parvalbumin neurons, enhance cortical synchronization, and improve cognition in schizophrenia.