In neuropsychiatric conditions, cortical-basal ganglia distributed circuits are of interest because the output from corticostriatal-thalamic circuits targets prefrontal cortical cognitive and limbic regions, as well as frontal motor regions, areas that allow the basal ganglia to modulate nonmotor, cognitive, and emotional functions (
1–
3). The striatum can be divided into three functional zones—limbic striatum, associative striatum, and sensorimotor striatum—all defined by their cortical connections. Each of these functional zones receives topographically organized tracts from the cortex forming three spatially and functionally segregated corticostriatal-thalamic feedback subloops. Furthermore, these striatal zones also receive spatially overlapping, functionally integrative tracts from the cortex (
4,
5). Abnormalities in any one of the gray matter core components, or in the various white matter tracts connecting the gray matter components of the three subloops, can disrupt the functional output of the entire subloop and thus directly alter cognitive, affective, or sensorimotor function (
6–
10).
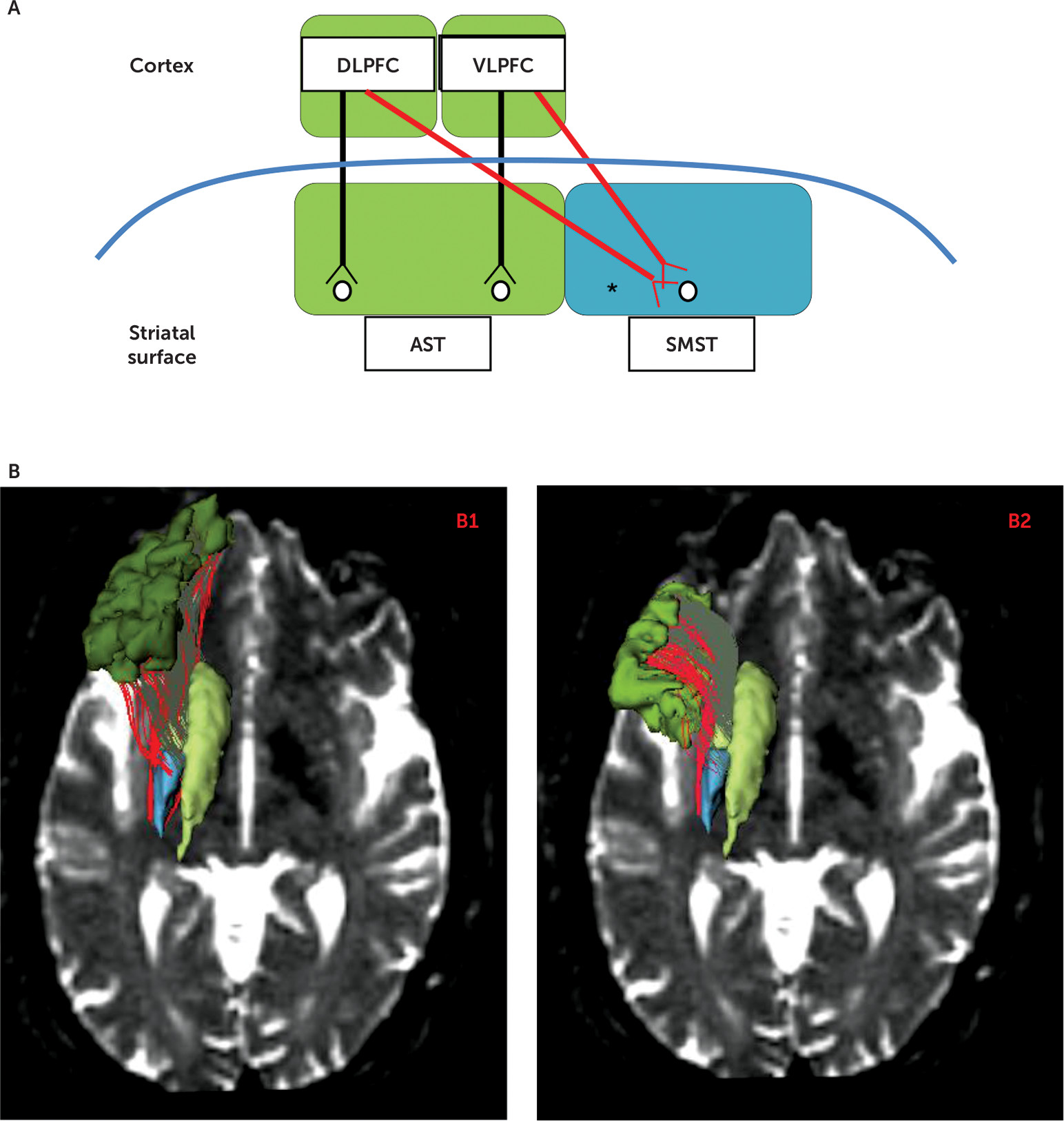
The present study focuses on white matter tracts in chronic schizophrenia patients and healthy control subjects that connect both the associative striatum and the sensorimotor striatum to the lateral surface of the cognitive associative cortex, key components of the associative and sensorimotor basal ganglia loops. The corticostriatal circuitry in normal brains is parsed into segregated versus integrative white matter pathways that project from the cortex to the striatum. Segregated tracts are those tracts that topographically connect functionally corresponding regions in the cortex and striatum (e.g., associative cortex to associative striatum), whereas integrative tracts connect functionally noncorresponding regions between the cortex and striatum (e.g., associative cortex to sensorimotor striatum) (
Figure 1A). Such an organization is strongly supported by both animal tract tracing studies and human imaging studies (
4,
5,
21). The distinction may have clinical importance because it has been proposed that the function of segregated tracts and their striatal target regions is to refine a skill already learned, whereas the role of the integrative tracts and their target regions is to allow for new, reward-based learning (
5,
22). More broadly, striatal subregions that receive both segregated and integrative inputs from the prefrontal cortex may play a role in integrating diverse prefrontal cognitive functions (
23–
25).
Using diffusion-weighted imaging tractography in chronic schizophrenia patients and healthy control subjects, we investigated fractional anisotropy and streamline counts, measures of brain region structural connectivity, in associative loop tracts connecting the prefrontal cortex and the striatum. Additionally, in all participants, we investigated the association between these measures of structural connectivity and executive function as assessed using the Trail-Making Test, Part B, a measure of executive function cognitive control (i.e., the ability to resist interference while pursuing a goal-directed task). First, we hypothesized reduced fractional anisotropy and number of streamlines, reflecting reduced corticostriatal connectivity in tracts connecting the prefrontal associative cortex with the associative and somatosensory subregions of the striatum in chronic schizophrenia patients. Second, we hypothesized an association between reduced structural connectivity in these tracts in chronic patients and in healthy control subjects and worse performance on the Trail-Making Test, Part B. Third, we hypothesized an imbalance in the type of corticostriatal tract input in chronic schizophrenia patients compared with healthy control subjects, with more pronounced group differences in integrative compared with segregated type tracts. We base this last hypothesis on the idea that schizophrenia has been described as a condition with impaired integration of thought, emotion, and behavior (
27), for example, as demonstrated by classic symptoms such as flat or inappropriate affect. Furthermore, since cognitive impairments, including learning new material, are characteristic of schizophrenia (e.g., see reference
28), we hypothesized that a deficit in integrative tracts that might underlie such impairments would be even more pronounced in the schizophrenia patient group than a deficit in segregated tracts.
Discussion
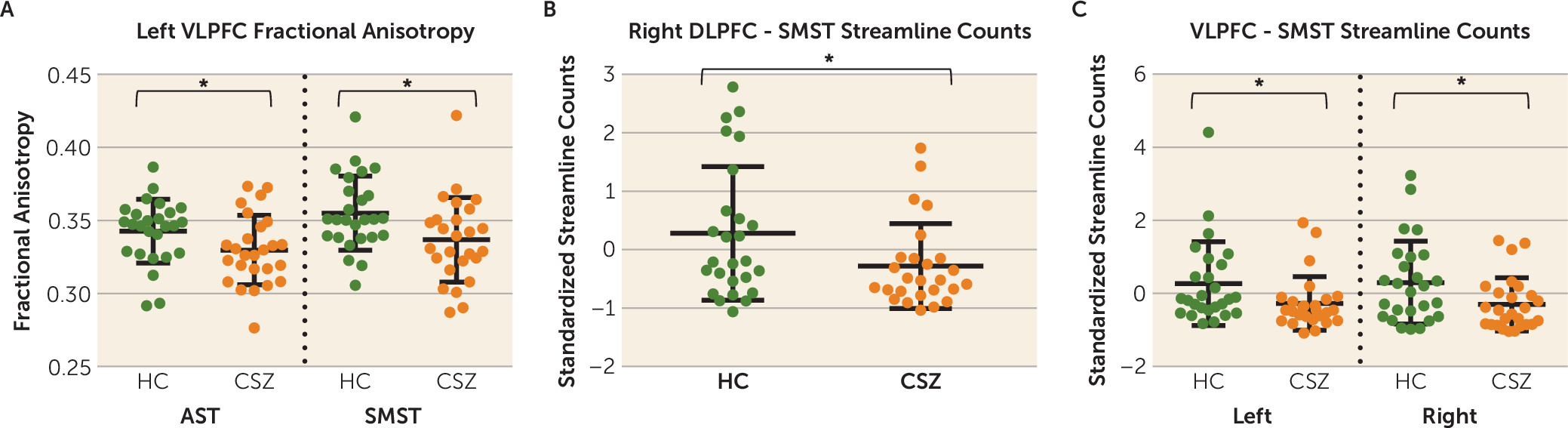
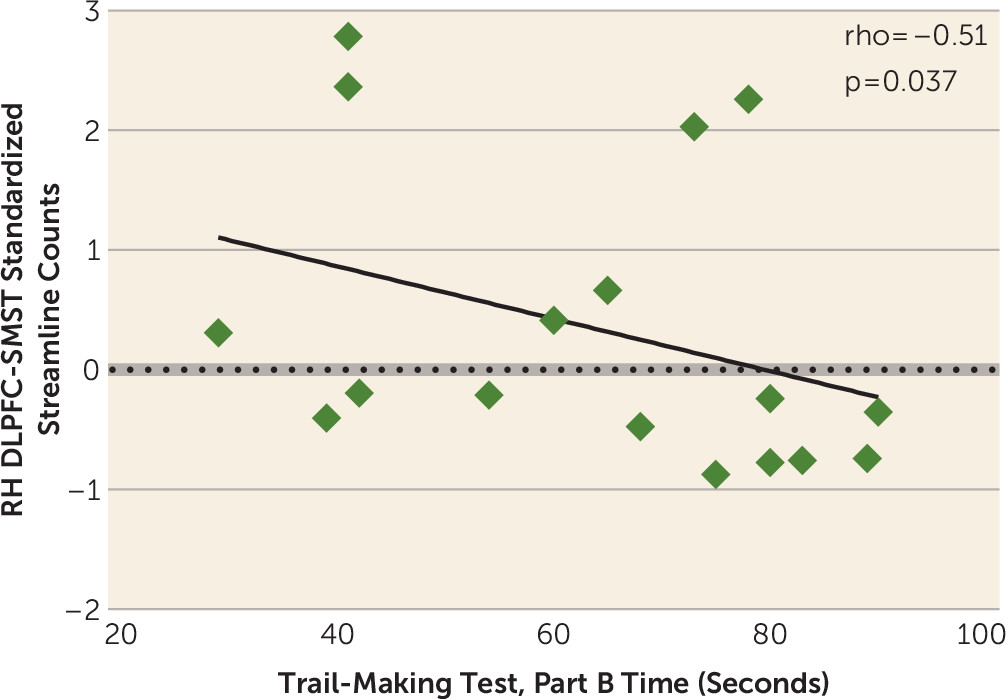
There are three principal findings in this study. First, we show reduced structural connectivity between the dorsolateral and ventrolateral associative cortex and the striatum in chronic schizophrenia as manifested by reduced fractional anisotropy, a measure of microstructural white matter integrity, and reduced normalized streamline counts, a measure of anatomical, macrostructural connectivity. Specifically, we show reduced fractional anisotropy in the left-hemisphere ventrolateral prefrontal cortex-associative striatum segregated tract and in the left-hemisphere ventrolateral prefrontal cortex-sensorimotor striatum integrative tract and a reduction in normalized streamline counts in the right-hemisphere dorsolateral prefrontal cortex-sensorimotor striatum segregated tract and in the left- and right-hemisphere ventrolateral prefrontal cortex-sensorimotor striatum integrative tracts. Second, in healthy control subjects, but not in chronic schizophrenia patients, we show that reduced streamline counts, corrected for intracranial contents, in the right-hemisphere dorsolateral prefrontal cortex-sensorimotor striatum integrative tract predicted worse performance on a measure of cognitive control, the Trail-Making Test, Part B. Third, against our prediction, we did not show a more pronounced group difference in integrative compared with segregated tracts (i.e., an imbalance in the degree of disruption in the type of corticostriatal tract input in chronic schizophrenia patients compared with healthy control subjects).
Our finding of reduced striatal structural connectivity in the associative loop in schizophrenia is noteworthy because the associative loop has gained in importance in understanding the pathophysiology of schizophrenia. For example, it has been demonstrated with positron emission tomography studies that there is excessive presynaptic release of dopamine both in acutely exacerbated chronic schizophrenia patients and in prodromal schizophrenia patients, which occurs, in particular, in the associative striatum subregion of the striatum (
12–
14).
It is also noteworthy that an important glutamatergic-dopaminergic interaction occurs at corticostriatal terminals in the brain. It has been shown that dopamine release at corticofugal glutamatergic synapses in the striatum decreases corticostriatal glutamate release via D
2 receptors, which serve as inhibitory presynaptic heteroreceptors on corticostriatal terminals (
48). Thus, decreased frontostriatal input from glutamatergic tracts projecting to the striatum in schizophrenia combined with excessive presynaptic dopamine release at the striatum in schizophrenia might compromise information processing via diminished corticostriatal glutamatergic neurotransmission in frontostriatal pathways in schizophrenia. Moreover, since increased dopamine release in schizophrenia targets the associative striatum in particular (
13,
14), one could speculate further that disrupted processing of information through corticostriatal loops would especially affect the associative loop and cognitive function influenced by this loop, such as executive function cognitive control. Further confirmation of this potential interaction between glutamate and dopamine is needed in future studies.
Behaviorally, we found poorer performance on both the Trail-Making Test, Part A, and Trail-Making Test, Part B, and in the change scores, in schizophrenia patients compared with healthy control subjects. By subtracting Part A time scores from Part B time scores, we adjusted for an individual’s processing speed and isolated the extra time it took an individual to perform a similar sensorimotor task but with the added cognitive complexity of Part B. Part B represents a dual-attention task shifting between numbers and letters. It requires the use of cognitive control with resisting interference. In order to perform the task speedily, the participant needs to rapidly inhibit the default response to go from a number to the next highest number and similarly to go from a letter to the next highest letter. Our data show that schizophrenia patients had even more difficulty performing the more cognitively challenging Part B task because there was a group-by-task interaction. Of importance regarding our brain-behavior correlations, it is the Part B cognitive control task and not the Part A processing speed task that correlates with frontostriatal connectivity in healthy control subjects.
This brain-behavior relationship is consistent with anatomic studies in rodents and humans linking the associative striatum to goal-directed behavior (
16–
18), which requires cognitive control. That we found this correlation in healthy control subjects but not in schizophrenia patients may suggest that the disrupted frontostriatal connectivity that we demonstrate in schizophrenia may also disrupt brain-behavior associations that should be present. That is, there is a loss of a brain-behavior association in schizophrenia that should be present were there not diminished connectivity in this key neural pathway for goal-directed behavior.
Moreover, that we found a correlation between normalized streamline counts in the dorsolateral prefrontal cortex-sensorimotor striatum integrative tract and performance on the Trail-Making Test, Part B, suggests that the dorsolateral prefrontal cortex mediates its effect on this cognitive control task through its projections to the sensorimotor subregion of the striatum, an integrative tract. That our correlation occurred with this tract emphasizes the potential behavioral importance of functionally integrative, corticostriatal connections. Integrative tracts project to striatal subregions that also receive tracts from other functional cortical areas. For example, the sensorimotor striatum receives functionally segregated tracts from the sensorimotor cortex and functionally integrative tracts from the associative cortex (
Figure 1A). Indeed, Averbeck et al. (
23) have suggested that portions of the striatum that receive input from multiple prefrontal cortical regions might serve as connection hubs where higher-level cognitive processing and integration of information from multiple systems can occur. In a similar vein, Haber and Calzavara (
25) have noted that a requirement for learning is the “integration of inputs related to emotional, cognitive, and motor cortical functions.” Furthermore, they have proposed that parallel and integrative tracts in combination permit “coordinated behaviors to be maintained and focused (via parallel networks), but also to be modified and changed according to the appropriate external and internal stimuli (via integrative networks).” That is, maintaining acquired skills might occur in striatal regions receiving parallel, segregated corticostriatal inputs, whereas adaptation and learning new skills might occur in hub regions receiving converging, integrative corticostriatal inputs. Lastly, that our finding was in the right hemisphere is logical in that Parts A and B of the Trail-Making Test are visual scanning attention tasks, and visuospatial attention is believed to be a right-hemisphere function (
33).
Furthermore, the frontoparietal cognitive control network, which is a neural substrate for cognitive control tasks such as the Trail-Making Test, Part B, is comprised of nodes that include the dorsolateral prefrontal cortex and the inferior parietal cortex based on resting-state functional MRI (fMRI) functional connectivity analyses (
49). Additionally, Choi et al. (
50), again based on resting-state fMRI, showed that the dorsolateral prefrontal cortex is functionally connected (i.e., temporally correlates, or coactivates) with both the associative striatum in the dorsal caudate and with the sensorimotor striatum in the posterior putamen. Because the dorsolateral prefrontal cortex and the sensorimotor striatum (the posterior putamen) are both components of the frontoparietal control network, disrupting structural connectivity between them (i.e., in the dorsolateral prefrontal cortex-sensorimotor striatum tract) would be expected to impair cognitive control performance, which is what we show in our healthy control subjects. Moreover, in a small, longitudinal study of individuals at ultra-high risk for psychosis who were scanned twice, it was reported that those ultra-high risk individuals who developed psychosis by scan 2 showed an increase in dopamine synthesis capacity in the sensorimotor striatum (
51). This study additionally suggests that the sensorimotor striatum, as well as associative striatum, is a potential region of interest in schizophrenia for further investigation.
It is noteworthy that in the present study, tracts projecting from the sensorimotor cortex to the sensorimotor striatum were not explored, since we previously showed diffusion abnormalities in pathways projecting from the associative cortex to the whole, undivided striatum in first-episode schizophrenia (
15). Here, we therefore similarly focused on associative, but not sensorimotor, cortical projections to the striatum. Nonetheless, considering the present findings that show diffusion abnormalities in associative cortical projections to the sensorimotor striatum in chronic schizophrenia, it would be of interest in a future study to also examine tracts from the sensorimotor cortex to the sensorimotor striatum.
Limitations of this study include the potential confound of neuroleptic medication, including that of cumulative antipsychotic exposure, on our measurements of frontostriatal tracts. However, since axons do not express receptors targeted by neuroleptic medication, such as dopamine and 5-HT receptors (
52), the structural effect of neuroleptic medication on white matter pathways is not readily apparent. Future studies of white or gray matter in schizophrenia patients should differentiate between those taking atypical neuroleptics and those taking typical neuroleptics, although we point out that not all atypical neuroleptic medications resemble one another in their effect on gray matter brain structures (
26,
53,
54). Moreover, to lessen the confounds of medication and illness chronicity, these studies should be replicated in early-onset and neuroleptic-naive prodromal schizophrenia patients, as well as in longitudinal studies that enable the assessment of the trajectory of brain changes in schizophrenia over the course of the illness.
A further potential limitation of our study is that in order not to reduce power, we did not explore all potential non-illness-related variables as covariates unless they reached a p value <0.05, which prevented us from fully exploring all potential covariates.
In summary, we found reduced frontostriatal structural connectivity in the associative loop of the striatum in chronic schizophrenia as reflected by a reduction in fractional anisotropy and streamline counts in both functionally segregated and integrative pathways. Such diminished frontostriatal connectivity in the associative loop in combination with hyperdopaminergia targeting the associative loop may especially compromise associative loop information processing in schizophrenia. In addition, we report in healthy control subjects, but not schizophrenia patients, the novel brain-behavior correlation that increased streamline counts in the right-hemisphere associative loop dorsolateral prefrontal cortex-sensorimotor striatum integrative tract predicted better performance on the Trail-Making Test, Part B, cognitive control measure. This finding is consistent with numerous studies in both rodents and humans demonstrating a role for the associative striatum in goal-directed behavior.