Attention deficit hyperactivity disorder (ADHD) is a common neuropsychiatric disorder affecting 5% of school-age children (
3). In addition to the theoretically proposed involvement of prefrontal and striatal regions (
4), neuroimaging studies have identified other brain regions that reportedly differ in ADHD, including the parietal and temporal cortices, the corpus callosum, and the cerebellum (
5–
8). Reduction in whole gray matter volume has been reported in ADHD (
9–
11), possibly reflecting alterations to typical development (
12). A recent meta-analysis reported white matter microstructure differences in the internal capsule, corona radiata, and cerebellum (
13) that appear to vary with symptom severity (
14). Differences in cerebral structure may give rise to functional alterations in ADHD, with dysfunctional regions evident across multiple neuronal systems involved in both higher-level cognitive functions and sensorimotor processes (
15). This has led to the characterization of ADHD as a structural and functional disruption of distributed brain networks (
15,
16).
Although ADHD is increasingly considered a disorder of brain networks, variations across tissue types and imaging modalities are often considered in isolation. During childhood and adolescence, gray matter volume, cortical thickness, and white matter microstructure develop along different trajectories (
19). These changes do not occur in isolation, and system-specific correlation patterns are apparent between cortical gray matter and underlying white matter connectivity (
20). Although the neurobiological mechanisms of this precise interplay are not well understood (
21), recent advances in MRI analytical methods allow for the modeling of shared variance across multiple neuroimaging modalities to better characterize neuroanatomical variability across tissue types and among individuals (
22). By providing multiple viewpoints of a shared physiological process, these techniques have the potential to increase the sensitivity to detect patterns of neuroanatomical variation in clinical populations and aid in the interpretation of such patterns.
ADHD is a multifactorial disorder with diverse associated risk factors, a range of comorbidities, including both internalizing and externalizing disorders (
26), and various patterns of impairment, including poor academic performance (
27), impaired social functioning, and delayed maturation relative to peers (
28,
29). To disentangle the effects of these complex and overlapping factors and to improve our understanding of heterogeneity in ADHD presentation, we combined a dimensional approach (across ADHD and control subjects together) with multivariate image statistics in an extensively phenotyped pediatric cohort (
30) to test the hypothesis that phenotypic variation is linked to specific alterations in brain structure.
By combining independent component analysis with canonical correlation analysis, we aimed to identify independent multimodal neuroimaging patterns, each associated with particular clinical and cognitive profiles. Finally, we tested the hypothesis that these markers can predict phenotypic variation in an independent cohort.
Discussion
In a data-driven analysis of multimodal imaging data and multi-informant phenotypic data, we identified a novel set of brain imaging patterns that reflect phenotypic variation across a cohort of children with ADHD and non-ADHD control subjects. Our results highlight how multiple cognitive and clinical variables can be factored to explain observed behaviors. Importantly, the inclusion of neuroimaging data allows the identification and separation of the neurobiological correlates of these factors. Patterns across multiple anatomical scales were associated with developmental, clinical, and cognitive profiles. We found that these patterns proved informative to models of phenotypic variation in an independent pediatric cohort.
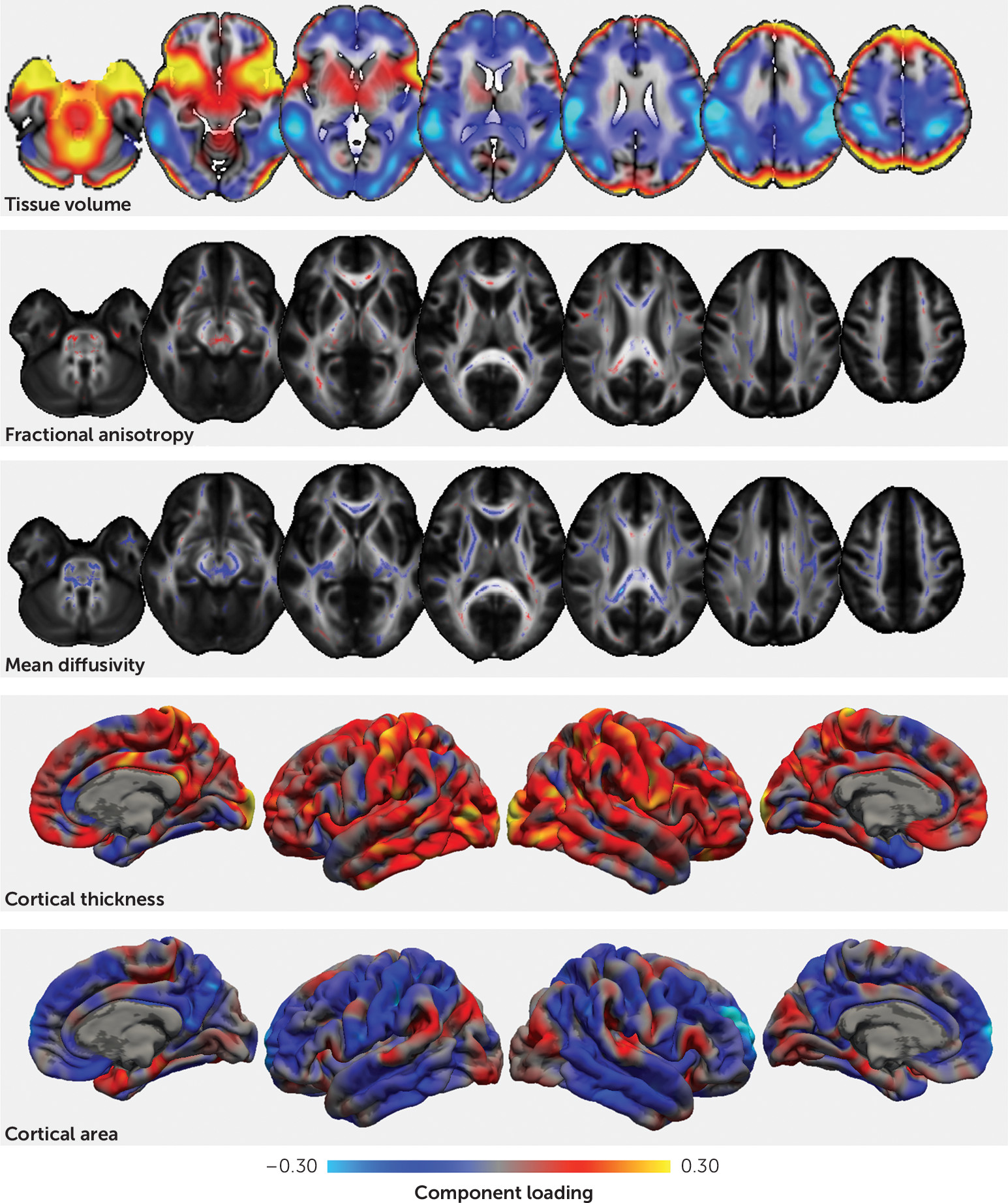
The first imaging pattern captured the well-known relationship between brain volume and cortical surface area (
39). Intracranial volume was higher in males, and individuals with higher intracranial volume scored well in mathematics and reading, lending support to recent reports of the shared genetic etiology between brain size, cognitive ability, and educational attainment (
40). This pattern was positively correlated with socioeconomic status, mirroring previous reports (
41). Contrary to evidence that intracranial volume is reduced in ADHD (
9,
18), ADHD symptoms were not significantly associated with this imaging marker. However, given the relatively small effect size of this reported difference, this is perhaps not surprising (
18).
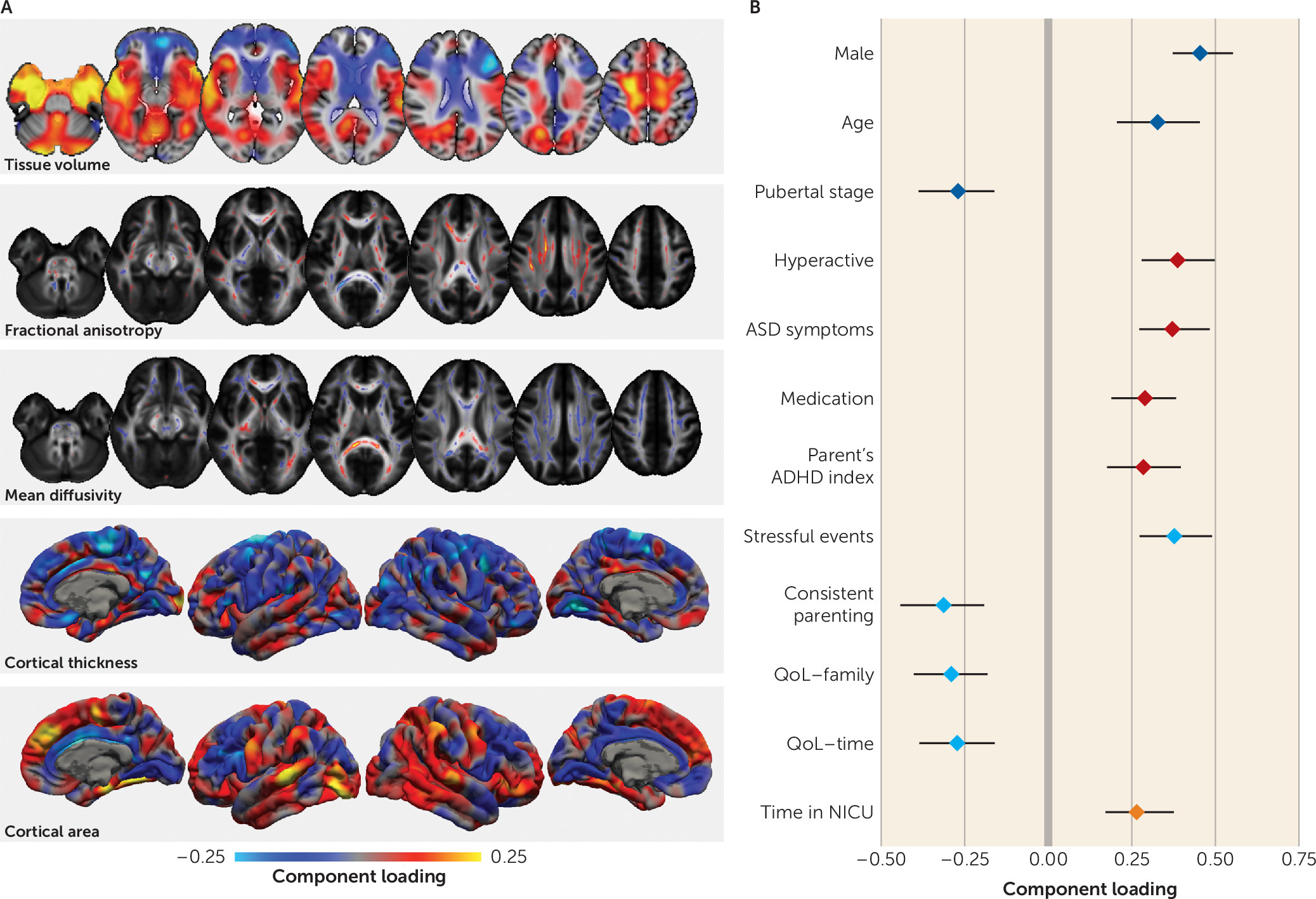
The second pattern captured neuroanatomical variation associated with childhood development indexed by greater body weight, pubertal status, and age. During puberty, longitudinal studies report decreases in cortical thickness with increasing circulating testosterone (
42). However, in prepubertal cohorts, dehydroepiandrosterone (DHEA), rather than testosterone, is associated with brain growth. DHEA levels have been associated with increased cortical thickness in the dorsolateral prefrontal cortex, the premotor cortex, and the temporoparietal junction in both males and females (
43). We previously showed that pubertal onset is associated with increased fiber density in the corpus callosum (
44). In the present study, we found a pattern of lower mean diffusivity evident in both the corpus callosum and the ascending white matter tracts. We postulate that the changes reported here reflect a combination of pubertal processes in addition to age-related variation.
We also observed a significant association between the second phenotypic factor and medication status, suggesting that younger, less pubertally mature children were more likely to be receiving medication for ADHD. While this is broadly consistent with epidemiological evidence showing that younger age relative to peers increases the likelihood of ADHD diagnosis and subsequent medication (
28), we previously reported that there is little evidence to support a simple correlation between age and diagnosis in this cohort (
45). Indeed, Table S3 in the
online supplement shows that although a lower score for this factor is associated with a slight increase in hyperactive symptoms (loading=−0.17), it explains only a small amount of variance in hyperactive symptom count (approximately 2%) and it occurs independently of other clinical factors. We propose that this pattern instead represents a marker of lower neuroanatomical maturity—or “brain age” (
46)—that is associated with both age and pubertal status and may manifest as a more immature behavioral profile characterized partly by increased hyperactivity.
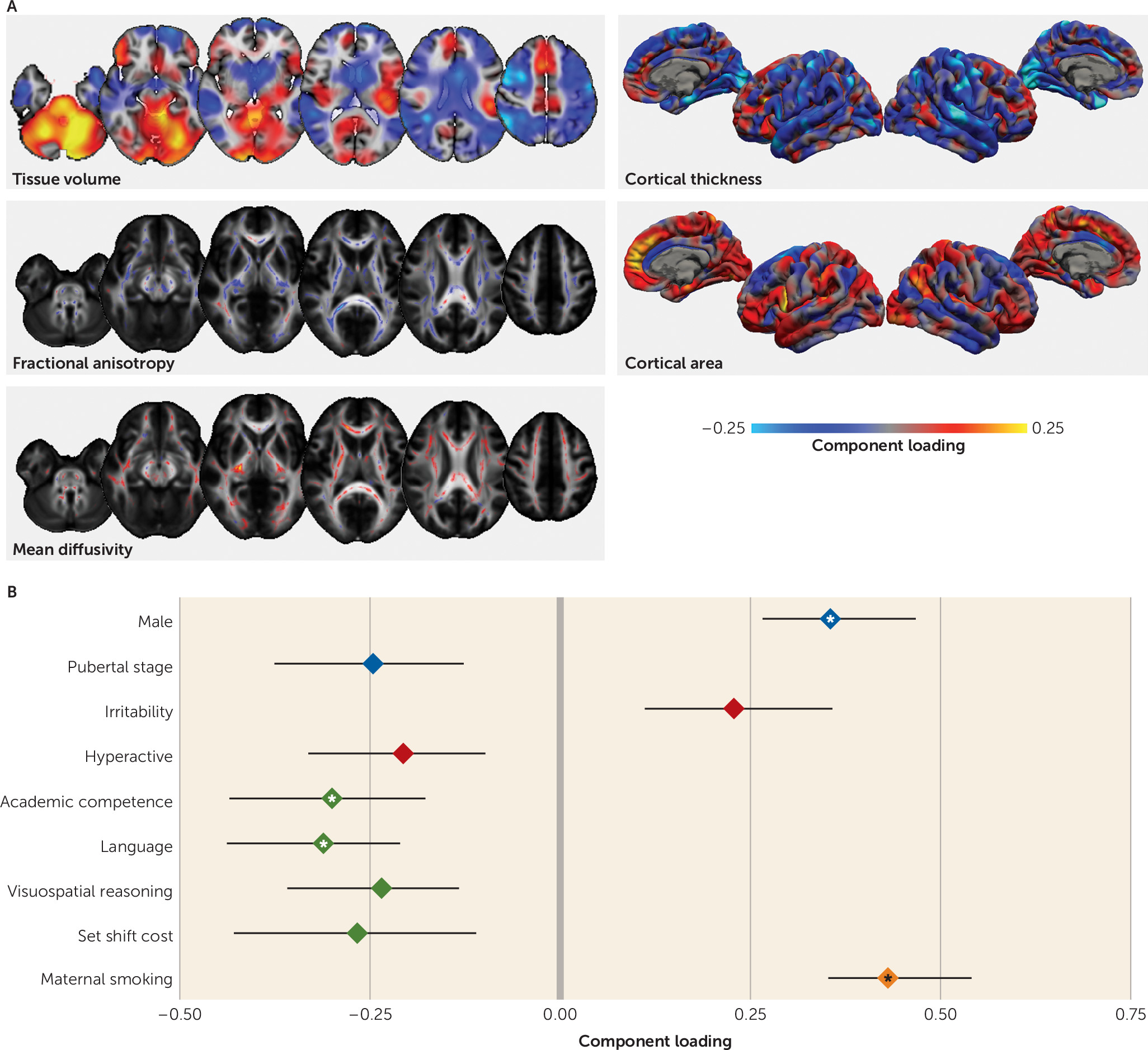
The third imaging pattern was correlated with a clinical profile marked by higher hyperactive symptom count, higher teacher and parent ratings of ADHD behavior, and more comorbidities. This profile was more common among male participants, who were less pubertally developed. It also correlated less with inattentive symptoms than with hyperactive symptoms, suggesting some domain specificity.
Previous studies comparing individuals with ADHD with control subjects have reported similar neuroimaging differences in clinical populations, including volumetric decreases in the anterior cingulate cortex and increases in the temporal lobe and around sensorimotor regions that covaried with hyperactive symptom severity (
23,
24). Other univariate approaches have yielded differences in frontal, parietal, and subcortical structures consistent with the patterns described here (
9,
10,
47). Our results also highlight the role of the superior longitudinal fasciculus, a structure that has been implicated in ADHD (
48), particularly in relation to hyperactivity (
49).
By including a rich scope of phenotypic data, we found that this factor was also correlated with poorer academic performance, lower parental education, and an increased experience of stressful life events. Symptoms of inattention and hyperactivity have previously been shown to correlate inversely with academic performance (
27), and early life deprivation can result in neuroanatomical alterations that mirror those seen in ADHD (
50). Similarly, van der Meer et al. (
51) identified genetic variants associated with stress exposure and hypothalamic-pituitary-adrenal axis activity that predicted ADHD severity in childhood, explaining approximately 12.5% of variance in symptom severity. These findings highlight possible mechanistic pathways that may lead to altered developmental neuroanatomy in ADHD.
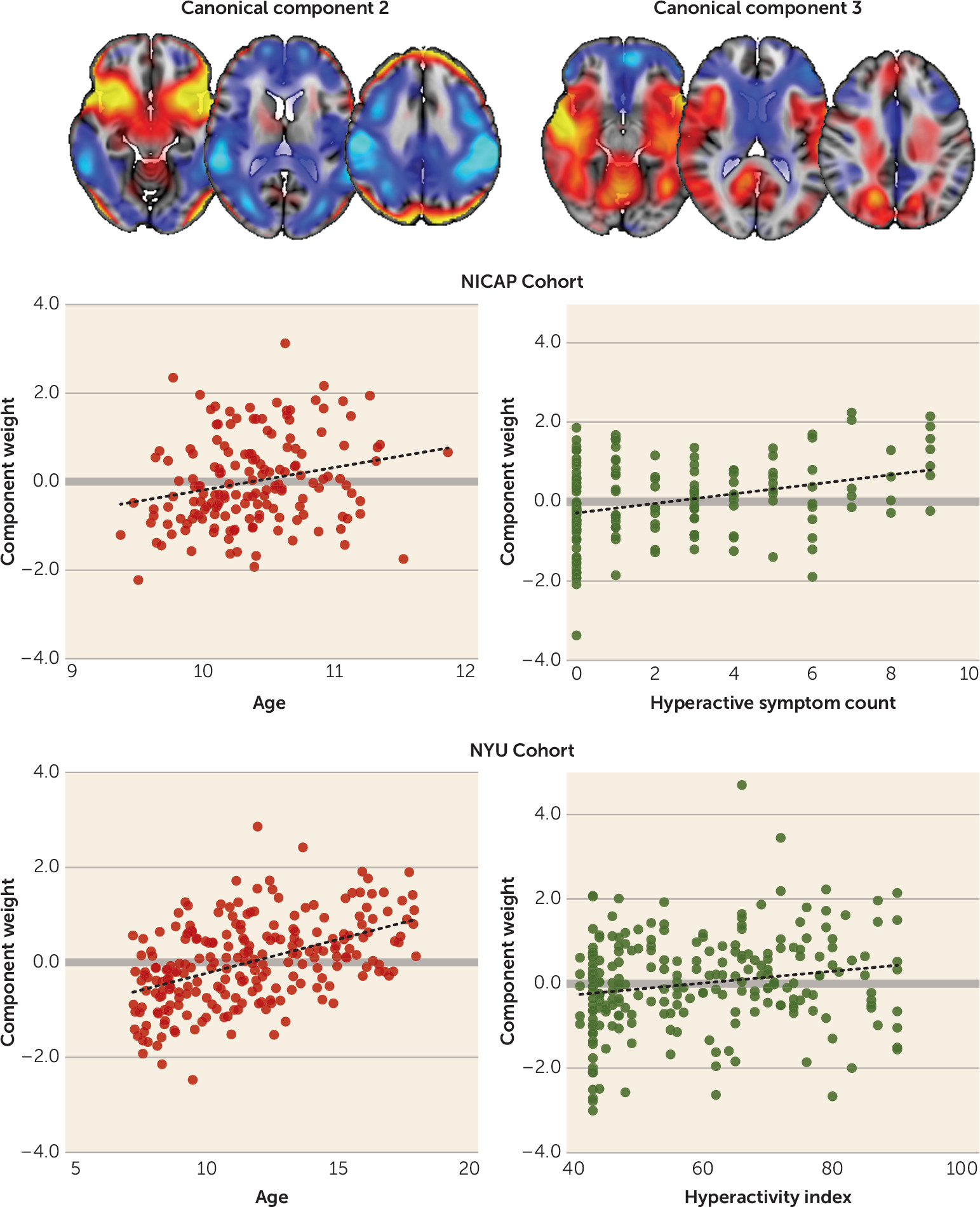
To demonstrate the external validity of our model, we estimated the expression of each imaging pattern in an independent cohort. We found a small but significant independent association between the ADHD imaging pattern and hyperactivity index in this validation cohort. In combination with age, sex, and IQ, this model explained approximately 10% of the variance in hyperactivity score in the NYU cohort. The imaging pattern alone explained 8% of variance in hyperactivity in the NICAP cohort. We also observed a measure of symptom specificity, with the imaging marker explaining approximately twice as much variation in hyperactivity than in inattentiveness in both the NICAP and NYU cohorts. The model compares well to previous reports. Recent large-scale genetic analyses have demonstrated that markers of genetic risk for ADHD explain around 0.1%–0.5% of the variance in ADHD trait behavior in the general population (
52). Furthermore, longitudinal studies find that neuropsychological assessment in preschool, socioeconomic status, and sex together explain around 8%–12% of variance in ADHD symptom severity in the same individual in later childhood (
53). With regard to MRI, Rosenberg et al. (
54) derived an imaging marker from functional MRI (fMRI) scans acquired during an attention task and found that this marker explained approximately 7%–9% of variance in ADHD symptom severity in an independent population.
The fourth imaging pattern was associated with a poorer cognitive profile but not IQ, and it highlighted the role of the dorsal attention networks and associated white matter organization for performance in visuospatial tasks (
55,
56). Although there were no broader cognitive variables available to test in the validation cohort, IQ was also not associated with expression of this marker in the NYU cohort. This suggests that this factor is not reflective of a general decrease in cognitive functioning but rather is specific to certain domains. Although some aspects of this factor structure proved less stable under model perturbation than the preceding three (see Figure S4 in the
online supplement), an environmental component may underlie cognitive performance, with lower parental education, maternal smoking, and a poorer quality of life associated with the cognitive profile. Clinically, the factor was associated with increased irritability and fewer hyperactive symptoms. Irritability is a common comorbid factor in ADHD, although this analysis suggests that its underlying neural correlates may be independent of ADHD, supporting a recent meta-analysis that suggests that irritability is a poor predictor of ADHD (
57).
In summary, these findings suggest that the presentation of ADHD may arise from a summation of several clinical, developmental, or cognitive factors, each with a distinct neuroanatomical foundation. Separate from the main clinical relationship, one factor reflected delayed development relative to peers, consistent with the theory of delayed maturation in ADHD (
29). Similarly, although irritability is often present in ADHD, it also had a presentation, independent of ADHD, associated with its own pattern of cognitive deficits and anatomical correlates. We anticipate that our work will inform future research into the neurobiological foundations of ADHD, highlighting the value of neuroimaging and the importance of leveraging detailed phenotypic data to understand the neurobiology underlying neurodevelopmental disorders.
Our study has a number of limitations that warrant consideration. The age range of the participants was relatively narrow, and the reported brain-behavior associations may vary across different stages of development. Future research in this longitudinal cohort will examine whether changes in these imaging markers predict longitudinal change in developmental, clinical, and cognitive phenotypes. Twenty-three individuals were taking medication for their ADHD, and the long-term effect of medication on brain structure may constitute a confounding factor. However, as medication may normalize neuroanatomical differences in ADHD (
58), such an effect would be expected to reduce the magnitude of the findings.
We plan in future work to incorporate functional brain measures derived from fMRI into this analytical framework. Co-occurring functional-structural deficits have been reported in ADHD using multimodal methods (
25), and fMRI networks are able to predict variation in attention and classify DHD subtypes (
54,
59). We anticipate that characterizing the functional correlates of the neuroanatomical patterns described here will prove informative to the theoretical modeling of the disorder.
In conclusion, this study furthers our understanding of brain development and mental health in childhood with evidence derived from multidimensional phenotypic and neurobiological measures. We identified four separable neuroanatomical patterns that are associated with unique developmental, clinical, and cognitive factors that span diagnostic criteria. We further demonstrated that the markers associated with development and an ADHD phenotype derived from a community sample can predict phenotypic variation in an independent cohort.