Violent and aggressive behaviors are highly prevalent among chronically hospitalized psychiatric patients. Of those patients who were in the New York State hospital system for longer than 1 month, 7% were assaultive at least once within a 3-month period.
1 In a 1-year period, approximately 12,000 assaults occurred in this hospital system.
2 In the Veterans Affairs hospitals, 12,000 assaultive incidents were reported over a 5-year period.
3 Patients with schizophrenia, mental retardation, and brain damage who are chronically hospitalized frequently exhibit aggressive behaviors.
4–6The first report of the use of beta-adrenergic receptor blockers in the treatment of aggression appeared in 1977. Since that time, more than 30 papers have appeared in the neurologic and psychiatric literature involving the use of beta-blockers in the treatment of violent and aggressive behaviors (see review
7). These papers report experience with approximately 300 patients, most of whom had been unsuccessfully treated with antipsychotics, minor tranquilizers, lithium, or anticonvulsants before a trial of beta-blockers was initiated. Several of these studies are prospective and controlled trials of the beta-blockers propranolol,
8–10 nadolol,
11,12 or pindolol.
13 Few studies have used objective ratings for the documentation of each aggressive episode; most have relied on modified scores of aggressive behavior that summarize this behavior on a weekly basis.
METHODS
Patients were hospitalized at either Creedmoor Psychiatric Center (CPC) in Queens, NY, or Middletown Psychiatric Center (MPC) in Middletown, NY. Patients at CPC resided on one of three hospital units: a specialized unit for highly aggressive patients, a unit for patients with severe mental retardation and behavioral problems, or a unit for long-term rehabilitation. At MPC, patients were on one of three long-term treatment units. Patients were included in the study if they were over 18 years of age, had at least a 6-month history of repeated verbal or physical aggressive behavior, and were diagnosed as having either a chronic psychotic disorder (schizophrenia or atypical psychosis), an organic mental disorder, or mental retardation. At CPC, aggressive behavior was monitored for at least 1 month before entrance into the study. At MPC, aggressive behavior of most patients was monitored for up to 1 year. If the aggressive behavior resolved during the baseline observation period, the patient was excluded from the study. Diagnosis was made through the use of a modified Schedule for Affective Disorders and Schizophrenia (SADS) interview,
18 a complete chart review, and an interview of available family members. Patients were excluded from the study if they had asthma, chronic obstructive pulmonary disease, diabetes mellitus, hypoglycemia, cardiac disease, peripheral vascular disease, or hyperthyroidism.
In accordance with the mental hygiene code of New York State at the time of the study, the treating psychiatrist for each patient determined whether that patient was competent to provide informed consent for participation in research. For those patients who were not competent, available family members provided consent. Where no family members were available, a designee of the director of the facility signed for the patient. The Institutional Review Boards of the New York State Psychiatric Institute, Presbyterian Hospital, CPC, and MPC approved this protocol.
Study Design
Aggressive behaviors of individual patients were monitored through the use of the Overt Aggression Scale for a period of 3 to 52 weeks before entrance into the study to confirm the presence of frequent aggressive behaviors.
14–16 After patients' acceptance into the study, their dosages of current medications were stabilized. Psychotropic and anticonvulsant medication schedules and doses were not changed during the study, unless required for management of side effects or toxic effects. For prn medications, only diphenhydramine, chloral hydrate, sodium amobarbital, and paraldehyde were permitted. This was done so that the dose of antipsychotic medications received through the study would remain unchanged.
During the first week of the study, placebo tablets were administered (one, four times a day [qid]). Propranolol then was initiated at a dosage of 20 mg qid. At CPC, the dosage was increased by 20 mg qid every 4 days; at MPC, the dosage was increased by 20 mg qid every other day until a dose of 400 mg/day was reached, and then increased by 20 mg qid every day. The dose was increased until a dosage of 1,440 mg/day or 20 mg/kg was reached (whichever was higher). Pulse rate and blood pressure were monitored in both the sitting and the standing position before the administration of each dosage of medication. If the patient had complaints of dizziness, ataxia, or wheezing, or had persistent pulse rate of less than 50 or systolic blood pressure of less than 90, the medication was not increased and was maintained at a dosage that was better tolerated. When required, the dosage of concomitant medication was decreased to permit upward adjustment of the propranolol dose. Whenever possible, patients were maintained on this dosage for a minimum of 2 months.
Patients who had an equivocal or definite clinical response, as defined by clinical global impression during the open trial, were eligible to enter into the double-blind placebo-controlled discontinuation phase of the study. (Clinical Global Impression Scale [CGI] change of 1 was defined as equivocal response; CGI change of 2 or greater was defined as definite clinical response.) Enrollment in this phase was not always possible because of patient noncompliance or refusal, transfer to another institution, or administrative difficulties within the institution. Patients were randomly assigned either to continue on propranolol or to have the propranolol discontinued by gradual substitution of identical-appearing placebo medication for the propranolol tablets. The number of pills administered remained constant throughout the double-blind phase of the study. Neither clinical nor research staff were aware of whether the patient had been assigned to be maintained on active drug or discontinued. At CPC, propranolol was tapered at a rate of 80 mg qid. At MPC, propranolol was tapered at a rate of 80 mg/day. Patients were observed for up to 2 months after the date when tapering would have been completed.
Psychiatric Evaluations
Aggressive behavior was evaluated biweekly through the use of the CGI and Global Assessment Scale (GAS).
19 Each aggressive episode that was observed was rated by the clinical staff (aides, nurses, social workers, psychologists) through the use of the Overt Aggression Scale. This scale has been described in detail elsewhere, along with demonstration of the validity and reliability of the scale.
14–16Biochemical Evaluations
Blood samples for plasma levels of antipsychotic and anticonvulsant medications were collected every 2 weeks during the open phase of the study and monthly thereafter. Antipsychotic medication plasma levels that were monitored included thioridazine, mesoridazine, chlorpromazine, haloperidol, and fluphenazine. Details of processing of the samples and determination of plasma levels have been described previously.
20Analysis of Data
All patients were included for efficacy analysis if they received propranolol for longer than 1 week. From the OAS, an aggression score (AS) and the number of aggressive episodes (AE) were calculated weekly for each patient. The AS is obtained by adding the weighted score for the most severe degree of aggression within each category of aggressive behaviors. The weighted score for the intervention was not included because we observed during the course of the study that for some patients, staff interventions changed after entrance into the protocol; for example, they gave fewer prn medications. For each patient, AS and AE were calculated for the month prior to receiving propranolol and, when possible, for the second month after the patient was receiving the maximum dose of propranolol. At CPC, this was done for all patients except for 2 who did not complete the open phase of the study. (One was transferred to another institution, and another dropped out due to hypotension.) At MPC, this was done for all patients except for 1 patient for whom it was necessary to decrease the dose of chlorpromazine during the trial because of hypotension and 1 patient who eloped from the hospital. Where applicable, AS and AE were calculated for the last month of the double-blind discontinuation phase of the study.
CGI and GAS ratings for these 4-week periods were averaged. OAS measures, CGI, and GAS scores were analyzed by paired t-test (two-tailed) for significant differences between ratings or scores before and after the open propranolol trial. Repeated-measures analysis of variance with study site as a covariate was performed to determine the presence of a treatment effect by Center.
At the conclusion of the study, all available data were examined, including AS, AE, CGI, GAS, and graphs of weekly AS to determine response. The patient population was divided into those who showed improvement in AS of greater than 50%, less than 50%, and no improvement or worsening of aggressive behavior. Results of the double-blind phase of the study were rated according to change in AS compared with the response during the open propranolol trial. The small number of patients did not permit statistical analysis.
RESULTS
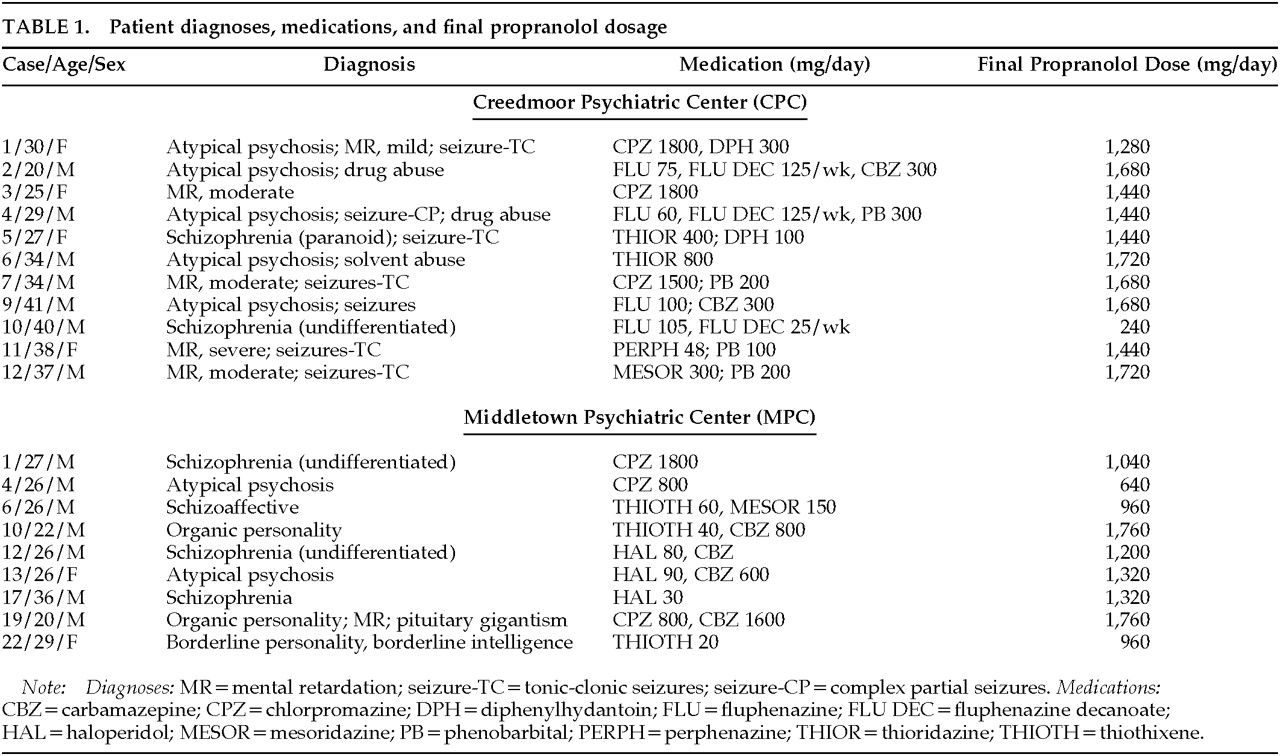
Of the 21 patients who entered the study, 20 received propranolol for longer than 1 week. One patient who was transferred from an intensive secure facility in New York for the purpose of this study was too violent to remain at CPC and was returned to the secure facility. The age, sex, diagnoses, antipsychotic and anticonvulsant medication, and final propranolol dosage for these patients are listed in
Table 1. There were 6 women and 14 men entered into the study. The diagnoses of these patients included atypical psychosis in 7, mental retardation (6), schizophrenia (5), schizoaffective disorder (1), drug abuse (3), seizure disorder (7), organic personality disorder (2), and borderline personality disorder (1). Antipsychotic drugs included chlorpromazine (6), fluphenazine (4), fluphenazine decanoate (3), thioridazine (2), thiothixene (4), perphenazine (1), mesoridazine (2), and haloperidol (3). Converting the dosage into chlorpromazine equivalents, the mean antipsychotic dosage was 2,598 mg/day (SD= 2,700 mg), with a range of 500 to 8,747 mg/day. This dosage calculation did not reflect the additional contribution of fluphenazine decanoate. Anticonvulsant drugs included carbamazepine in 6, phenobarbital (4), and diphenylhydantoin (2). Except for 1 patient who received only 240 mg/day of propranolol (because of hypotension), all other patients received at least 640 mg/day (mean dose 1,336 mg/day, SD=396 mg).
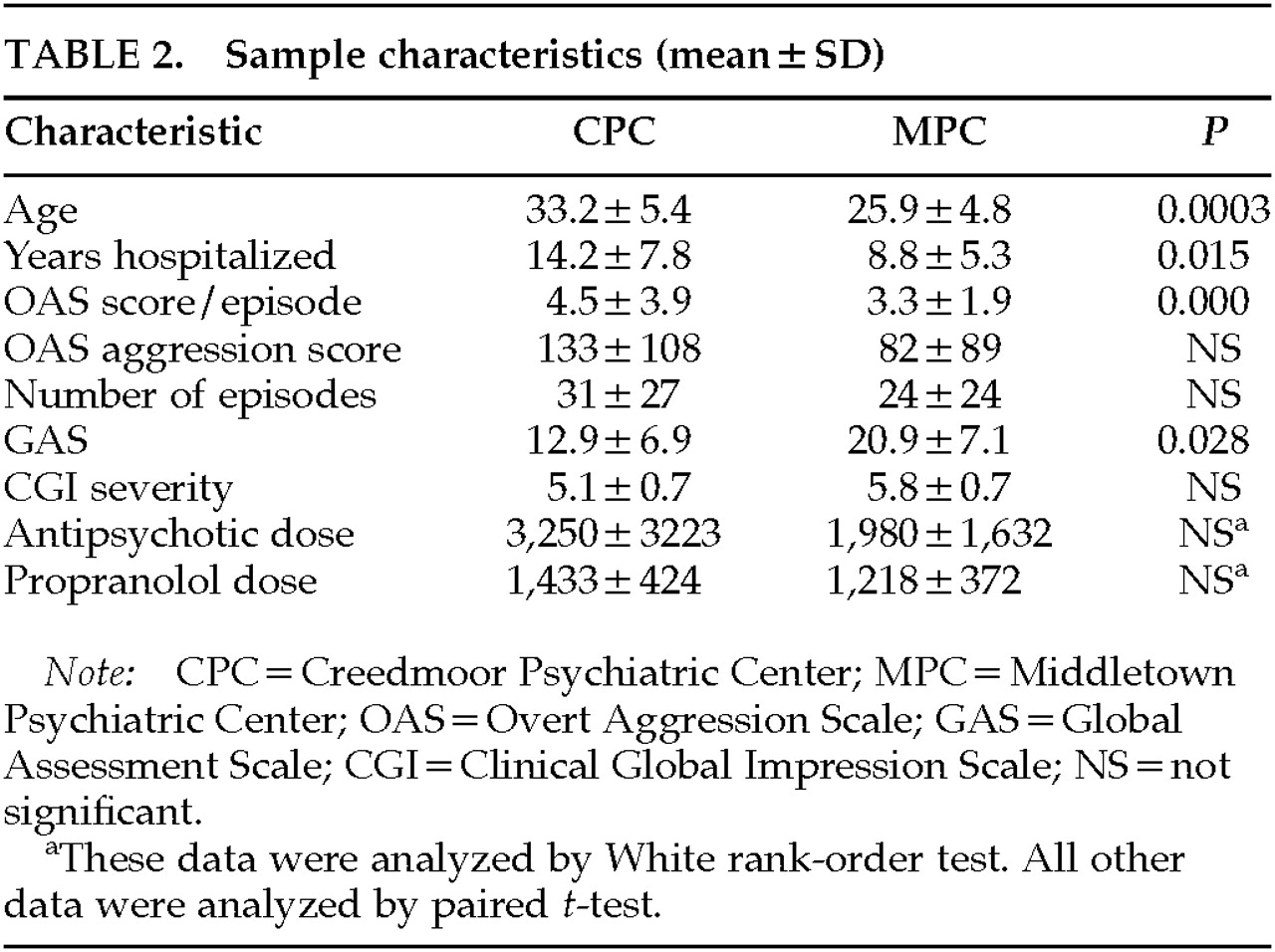
Table 2 compares the demographic characteristics of the patients at each hospital. The patients at CPC were significantly older and had been hospitalized for a longer period of time. The CPC patients had a more severe average OAS score per episode and a lower GAS. However, the monthly AS and AE and CGI severity were not significantly different between Centers. Because of the wide range of antipsychotic dosages administered, the two Centers were compared by using the White rank-order test. Although there was no significant difference in the dosages administered in the two Centers, there was a trend toward significance (
P=0.08).
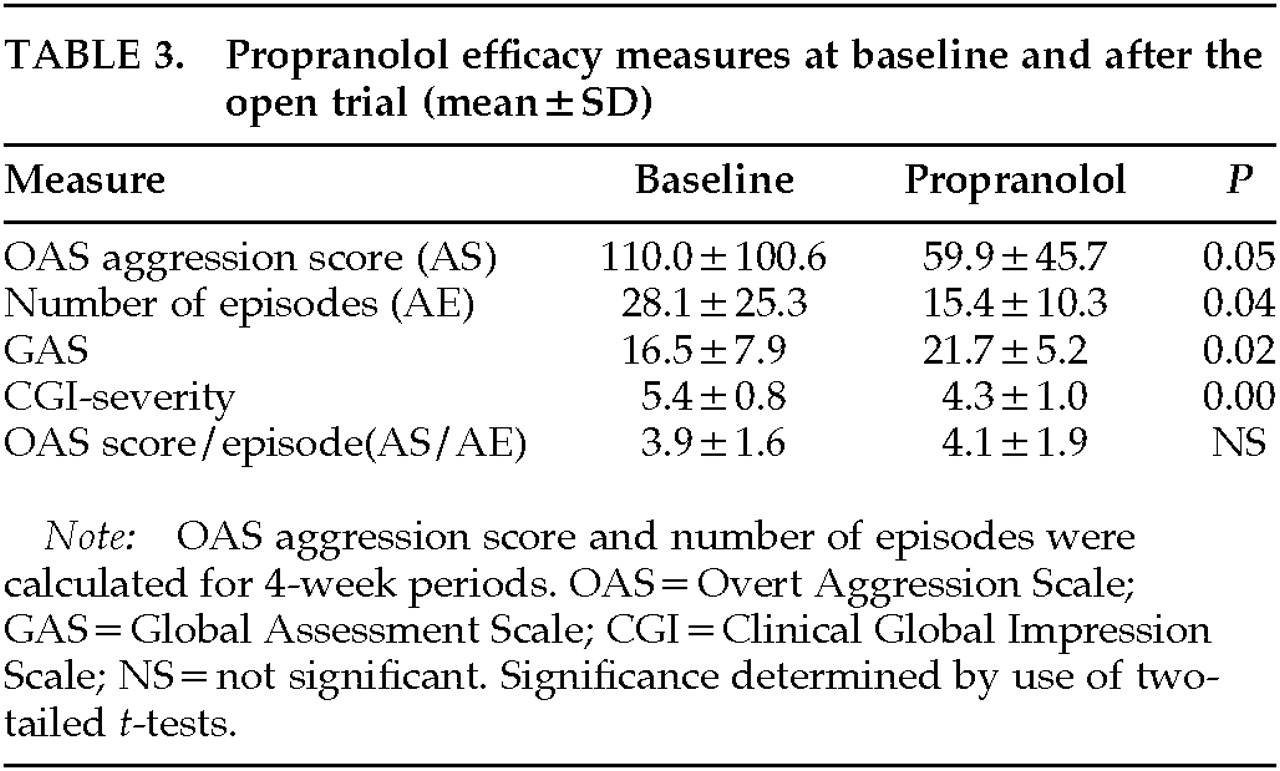
The data for efficacy measures at baseline and after the open trial are listed in
Table 3. Analysis of the open propranolol trial results indicated significant improvement for all four measures (AS:
t=2.09, SD=23.9,
P =0.05; AE:
t=2.16, SD=5.87,
P=0.04; GAS:
t=–5.17, SD=2.0,
P=0.02; CGI:
t=4.45, SD=0.24,
P=0.00). There was no change in the severity of each episode, calculated by AS/AE (
t=–0.46, SD=0.422, not significant), although there were fewer aggressive episodes during propranolol treatment. Repeated-measures analysis of covariance (controlling for Center) revealed no significant treatment by Center interaction.
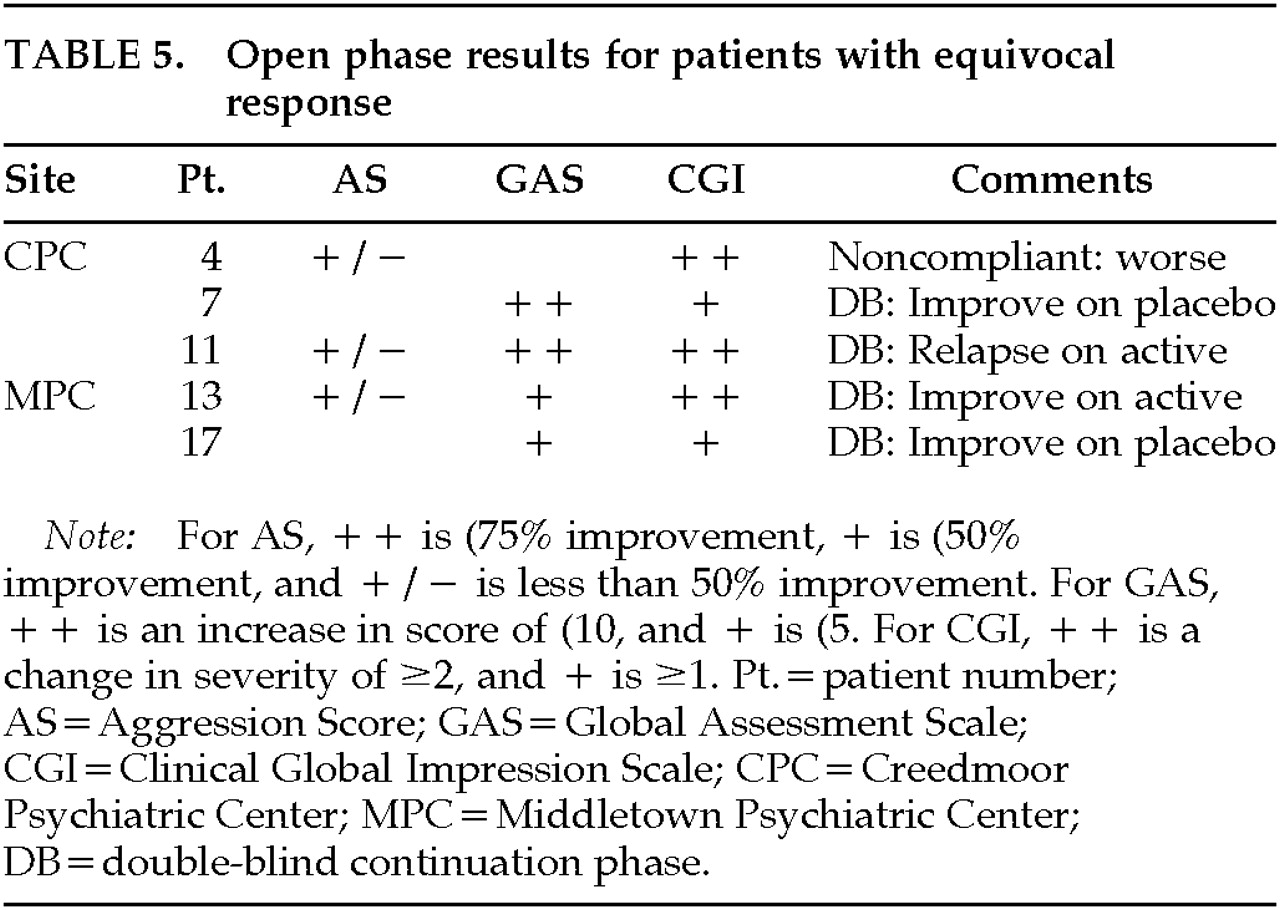
The open phase results of individual patients who were rated as exhibiting definite or equivocal response are listed for changes in AS, GAS, and CGI (
Table 4 and
Table 5, respectively). As an example of the improvement exhibited by individual patients, patient CPC 1 had 28 aggressive episodes during the baseline period (7 per week), and only 6 during a month on propranolol (1.5 per week). Patient CPC 19 had the number of aggressive episodes decrease from 86 per month (21.5 per week) to 7 per month (1.75 per week).
Of the initial cohort of 20 patients, 5 had a greater than 75% improvement on AS (CPC 1, 6, 9; MPC 12, 19), and 2 had a greater than 50% improvement (CPC 3, 5). Four of these patients were entered into the double-blind phase of the study (CPC 1, 6; MPC 12, 19). Two relapsed when switched to placebo (MPC 12, 19), one maintained improvement on active medication (CPC 1; propranolol), and 1 patient refused medication and relapsed, although he was being maintained on active medication (CPC 6). Of the other 3 patients, 1 was transferred to another facility (CPC 3), 1 became worse with open tapering of the drug (CPC 5), and 1 became worse when carbamazepine and propranolol were openly tapered (CPC 9).
Five patients exhibited improvement on at least one of the scales but showed less than 50% improvement in AS (CPC 4, 7, 11; MPC 13, 17). Of the 2 patients who showed no improvement or a worsening during the open phase (CPC 7; MPC 17), both showed improvement during the double-blind phase when switched to placebo. Of the other 3 patients, 1 relapsed when maintained on active (CPC 11), 1 continued to improve on active (MPC 13), and 1 relapsed when he became noncompliant (CPC 4). We consider that these latter 2 patients were equivocal responders to propranolol.
Importantly, 2 patients who were assessed as showing improvement on CGI (CPC 7 and MPC 17) exhibited no improvement when actual aggressive behaviors were examined, and 1 patient (CPC 3) who had more than a 50% decrease in AS had no improvement on CGI. Because of this observation, we examined the correlation among the efficacy assessments. Although AS and AE were highly correlated (r=0.96, P=0.00), AS was not correlated with either CGI or GAS.
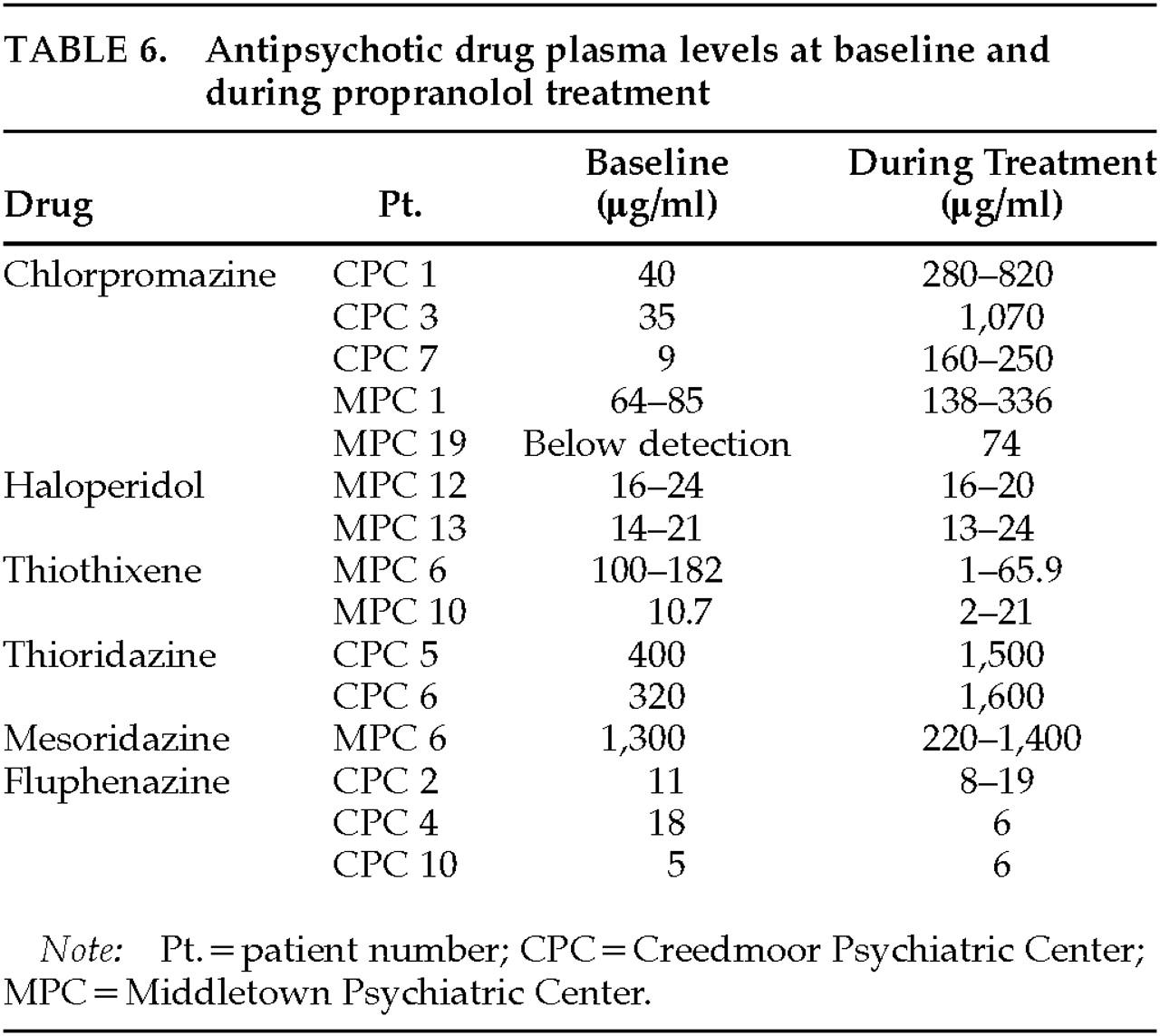
Psychotropic medication plasma levels are listed in
Table 6. Thioridazine and chlorpromazine levels increased during the propranolol open phase. The 2 patients who received thioridazine had increases in plasma levels into the potentially toxic range. This result has been reported in detail elsewhere.
20 Haloperidol and fluphenazine levels did not change. Although several patients who responded to propranolol exhibited increases in antipsychotic blood levels (CPC 1, 5, 6 and MPC 19), other patients who responded did not exhibit these increases (CPC 4, MPC 12). In addition, many patients with increases in antipsychotic levels did not respond (CPC 2, 7, 12 and MPC 1, 6, 10)
Side effects experienced by patients included dizziness in 7, nightmares (2), unsteadiness (2), fatigue or sedation (2), alopecia (1), increased urination (1), and asthma (1). Two patients had possible seizures while on propranolol. One of these patients had not experienced a seizure in the past.
DISCUSSION
This study of beta-blocking drugs in the treatment of aggression included both prospective design and the objective documentation of aggressive episodes. During the open phase of the study, approximately one-third of the patients had a greater than 50% decrease in aggressive behavior (aggression score and number of episodes per month). Because of the difficulties inherent in conducting a prolonged treatment study with chronically hospitalized patients, we were unable to complete the study with a sufficient number of subjects to statistically analyze the controlled phase of the study. The data available for the placebo-controlled phase confirmed that these patients responded to propranolol.
About one-third of these patients responded to propranolol in our study. We believe the most likely explanation for this refractoriness of aggression is the chronicity of aggression in our patients. After years of hospitalization, patients who are aggressive can have these behaviors reinforced by several factors. For example, the aggressive patient receives more attention from staff, either through interactions after specific episodes or transfer to “special” units that have a higher staff : patient ratio. The results of this study not only illustrate the clinical utility of the OAS for documenting change in aggression, but also seriously call into question the validity of global weekly or biweekly ratings. Such a procedure would include questioning the rating of aggressive behaviors with the OAS on a weekly basis, as has been proposed by some investigators.
21 It was our impression during the study that the reactions of clinical staff to the patient were dependent on how that patient was doing during the day or two before the rating. Aggressive behavior is by nature episodic, and analysis of change is comparable to the monitoring of seizures—rather than of psychosis or depression, states that are relatively constant from day to day. If a patient had an episode, staff tended to ignore how the patient was doing for the previous week. Estimated “weekly” ratings may not reflect actual aggressive behavior, since global ratings (CGI and GAS) may have changed in response to other factors, such as mood, irritability, apathy, or intensity of episode.
It was invaluable to have the aggressive behavior of some patients monitored for a prolonged time. This monitoring revealed that even severely aggressive patients have weeks of minimal or no aggression. Any study must control for the expected weekly and monthly variations in these behaviors. We believe that in a research study, the alternatives are the prolonged prospective monitoring of aggression on a daily basis or the inclusion of a large number of patients so that these fluctuations are balanced between treatment groups.
There are several possible explanations for decreases in aggressive behavior with propranolol treatment. Beta-blockers have been shown to decrease aggressive behavior in animals after both acute and chronic administration.
22,23 Weiler et al.
24 suggest that propranolol's effect results from an increase in presynaptic norepinephrine output and postsynaptic receptor density. Some investigators have suggested that beta-blockers may work peripherally by the reduction of afferent adrenergic stimuli.
25 In part, this proposal is based on the observation that nadolol, a nonlipophilic beta-blocker, may be effective in the treatment of aggression. However, as Gengo et al.
26 have pointed out, it is only when passive diffusion is the primary means of entrance into the central nervous system that lipid solubility predicts CNS penetration. All beta-blockers enter the CNS in pharmacologically sufficient concentrations. Leavitt et al.
23 have demonstrated that when nadolol is administered to 6-hydroxydopamine–treated rats by a route that has complete or predominant central effect (i.e., directly into the ventricles), there is a significant decrease in aggression.
It is unlikely that the decrease in aggression was a result of changes in the plasma levels of concomitantly administered medications. There were patients who showed decreases of more than 50% in aggressive behavior while having increases, or no change, in antipsychotic plasma levels, and there were also several patients who had significant increases in antipsychotic plasma levels and did not improve. Anecdotally, several patients required at least some antipsychotic medication to maintain their improvement. However, this was not done in a controlled, blind fashion. Past reports of the efficacy of beta-blockers in treating aggression have shown that many patients have improved on beta-blockers alone.
7 In addition, research suggests that high-dose antipsychotics are no more effective, and many actually increase aggression.
27Beta-blockers have been shown to treat neuroleptic-induced akathisia.
28 Some of these patients, many of whom were on high-dose antipsychotics, could have had aggression induced by akathisia, the latter being then treated with propranolol; although these patients did not complain of restlessness, we realize that this group of patients has difficulty in verbalizing affects. Previous reports, however, have demonstrated effectiveness of beta-blockers in patients who were not treated with antipsychotic medications.
7Several variables that figured in the study may have affected the results. These patients were chronically hospitalized, and therefore the results may not be generalizable to all aggressive patients. In addition, the patient population consisted of individuals with various diagnoses (e.g., mental retardation and schizophrenia). Theoretically, aggressive behavior exhibited in differing diagnoses could have a differential response to propranolol.
Because only a few patients entered the double-blind phase of the study, we were unable to statistically analyze continued response versus relapse. Propranolol was used in addition to concurrent medications, and therefore we cannot ascertain whether response was due to propranolol or to the combination of medications.
We used high doses of propranolol in order to ensure that all patients received an adequate trial. Propranolol was well tolerated in these patients, without significant adverse effects. The preponderance of published reports suggests that a dosage of 640 mg per day is usually effective. Beta-blockers should not be administered to patients with specific physical illnesses, such as bronchospasm, diabetes, thyroid disease, and heart failure. We did not observe any depression in our group of patients, and we believe that the occurrence of depression with propranolol treatment has been overstated.
29,30The results of this study are consistent with antiaggressive effects of propranolol in a cohort of violent, chronically hospitalized patients. Further studies of the effects of beta-blockers in the treatment of aggression are necessary. It is critical for future research efforts to include the objective documentation of aggressive behaviors so that specific criteria for response can be demonstrated.