Efforts to understand and prevent suicidal behavior have led clinical investigators to examine the cognitive styles and capabilities of suicidal individuals. Following the work of Aaron Beck and colleagues,
1 some investigators focused on cognitive “distortions” or “schemas” that might cause or perpetuate depression and suicidal behavior.
2,3 Another popular model proposed that “cognitive rigidity” mediates the relationship between stressful life events and suicidal behavior.
4–7 According to this view, individuals become suicidal in the midst of stressful life circumstances because of a rigid style of perceiving and reacting to the environment that makes it difficult to formulate alternative solutions to problems. “Cognitive rigidity” has been operationalized and assessed in a variety of ways, involving personality, interpersonal problem-solving, or neuropsychological constructs. Personality theorists have found that individuals categorized as rigid
8 or less open to experience
9 on standardized inventories were more prone to suicidal behavior. Schotte and Clum
5 found that suicidal individuals did more poorly on interpersonal problem-solving tasks.
Early neuropsychological investigations of the rigidity hypothesis provided some evidence that suicidal individuals had difficulty identifying, generating, and/or implementing divergent strategies to solve cognitive problems.
6,10 For example, Patsiokas et al.
6 compared a diagnostically heterogeneous group of 49 male patients admitted to a psychiatric hospital following a suicide attempt with 48 patients who had never attempted suicide. They found that the suicide attempters ages 19 to 50 years had more difficulty generating alternative uses for common objects (Alternate Uses Test).
11The work of Bartfai et al.
12 differentiated various aspects of problem-solving ability purported to be characteristic of the suicidal individual, but at the same time exemplified the methodological limitations encountered in this literature. They compared 9 diagnostically heterogeneous psychiatric patients who had attempted suicide within the past 3 weeks with 7 chronic pain patients and 8 healthy control subjects. The suicide attempters had more difficulty generating alternative solutions on measures of verbal and nonverbal fluency, although they were no different from control subjects on tasks assessing the capacity to recognize and respond to different solutions once these were provided. The investigation addressed neuropsychological specificity by including a range of measures, while at the same time using standardized diagnostic techniques to describe the patients. However, the samples were small and there was no control for contaminating factors such as substance abuse or depression.
To our knowledge, there are no neuropsychological studies of suicide that focus specifically on older adults, despite findings that older attempters differ from younger attempters in terms of psychiatric diagnoses, precipitating events, and medical comorbidities.
13–15 The scarcity of neuropsychological studies of older attempters is even more surprising given the age-related vulnerability to cognitive dysfunction, the well-documented associations between old age and lethality of suicidal acts,
16 and preliminary data suggesting an association between completed suicide and early Alzheimer's-type pathology in the old-old (age 71 or greater).
17 The present study was designed to address this critical gap in the literature.
We postulated that the suicidal behavior of older adults reflected age-related deficits in an array of complex cognitive abilities related to higher order thinking and problem-solving (“executive functions”).
18 Thus, we expected that with increasing age suicidal depressed individuals would have difficulty on measures of attention and concentration, abstract reasoning, the ability to alternate between two sequential tracks of information, and the rapid generation of novel responses to a problem (verbal and nonverbal fluency).
In light of considerable evidence of depression-related cognitive deficits in older adults,
19,20 we decided to control for depression by considering only depressed patients—that is, by comparing depressed suicidal and nonsuicidal patients. As well, we decided to exclude individuals with other types of psychopathology and medical illness that would be expected to impair cognitive function, such as schizophrenia, substance abuse, or neurological disease.
METHODS
Participants
Fifty-seven inpatients (40 females, 17 males) diagnosed with unipolar major depression by use of the Structured Clinical Interview for DSM-III-R (SCID)
21 gave informed consent to participate in a study of the neuropsychological effects of depression and suicide. Forty-four were included in a previous study of the neuropsychological effects of depression,
19 and all were included in a subsequent study of verbal learning of elderly depressed individuals.
22 Detailed descriptions of sampling methods were provided in those reports.
Eighteen of the depressed patients (10 women and 8 men) were included in the suicide attempter group (SAs) because they were admitted immediately following a suicide attempt (defined as an intentional self-damaging act). In 12 of 18 subjects, the index attempt was their first. The methods of attempting suicide included drug ingestion (9 participants), cutting or stabbing self (4 participants), carbon monoxide poisoning (2 participants), shooting (1 participant), drowning (1 participant), and other self-injurious behavior (1 participant). The SAs ranged in age from 50 to 84 years (mean=66.67±10.13 years; means±SD reported throughout), with educational levels ranging from 8 to 18 years (mean=12.56±2.91 years). Raw Vocabulary scores on the Wechsler Adult Intelligence Scale–Revised (WAIS-R)
23 were used as a gross estimate of verbal intelligence. The Vocabulary scores of the SAs ranged from 20 to 65 (mean=47.33±12.93). Scores on the 24-item Hamilton Rating Scale for Depression
24 (Ham-D) ranged from 17 to 46 (mean=28.06±8.36).
Of the 39 patients who had not made a current suicide attempt, 10 had attempted suicide in the past, and these were excluded from the study. This left 29 depressed nonattempters (NAs) who had no current or past history of suicidal behavior (20 women and 9 men). The age of NAs ranged from 50 to 86 (mean=64.24±10.97 years); years of education completed ranged from 8 to 18 (mean=12.66±2.14 years); and WAIS-R Vocabulary scores ranged from 15 to 59 (mean=41.66±12.21). Scores on the Ham-D ranged from 16 to 46 (mean=29.48±8.58).
On t-tests for unequal variance (α= 0.05), there were no significant differences between SAs and NAs in terms of average age, education, Vocabulary score, or Ham-D score. Although there were proportionately more men in the SA group, this difference was not statistically significant (Fisher's exact test, α=0.05).
Although 38 of the 47 patients were on psychotropic medications at the time of testing, our previous study of this sample detected no neuropsychological effects of these medications.
22 Moreover, there were no significant differences between SAs and NAs in terms of the proportion of patients using these medications (Fisher's exact test, α=0.05). A minority of the patients had electroconvulsive therapy (ECT) at some point in the remote past (more than 1 year prior to testing), but there were no significant differences between groups in terms of history of ECT (Fisher's exact test, α=0.05). Two of the SAs (11%) and 5 of the NAs (18%) were diagnosed with psychotic depression; all were carefully assessed to ensure that they could understand and respond to the test materials. These patients typically had somatic or depressive delusions; they did not have formal thought disorder that interfered with their cooperation during testing. There was no significant difference between groups in the proportion of patients with psychosis (Fisher's exact test, α=0.05).
Procedure: Target and Comparative Measures
Neuropsychological measures were administered within 1 week of admission, in a fixed order, as part of a larger protocol that assessed a broad range of functions.
19In order to examine the possibility that damaging effects of the suicide attempt itself contaminated our neuropsychological findings, we looked at the association between the medical lethality of the attempt (assessed with a standardized scale)
25 and the neuropsychological variables. There were no significant or near-significant relationships between the lethality ratings and neuropsychological scores (Pearson correlation coefficients, α=0.05).
Target Measures: We expected to find differences between SAs and NAs on the Attention/Concentration Index (A/C Index) from the Wechsler Memory Scale–Revised
26 (WMS-R); the Trail Making Test, part B (Trails B),
27 a test of tracking, sequencing, and mental flexibility; and the Wisconsin Card Sorting Test (WCST),
28 a test of abstract reasoning and “shift of set.” Trails B yields three scores used here: total time (Trails B Time), number of sequencing errors (Trails B Sequencing), and number of set loss errors (Trails B Set Loss). The Wisconsin Card Sorting Test yields two measures used here: total number of categories achieved (maximum possible=6) and number of perseverative errors (i.e., failure to shift set when indicated). As well, two types of fluency tests, “F-words”
29 and the Ruff Figural Fluency Test,
30 were included, following the work of Bartfai et al.
12 Comparative Measures: Although we did not expect suicidal depressed individuals to have impairments on these tasks, we included tests of confrontation naming,
31 verbal learning (total recall score from the California Verbal Learning Test),
32 constructional praxis (Block Design subtest of the WAIS-R),
26 complex copying (Rey-Osterrieth Complex Figure),
33 and visual integrative skills (Hooper Visual Organization Test)
34 for the purpose of comparison.
Statistical Analysis
To compare the cognitive performance of SAs and NAs relative to age, analysis of covariance (ANCOVA) was performed, using age, the raw Vocabulary score, and gender as covariates. Vocabulary was included as a potential contaminating variable in all of the analyses to control for the possibility that subtle group differences in verbal intelligence affected the results. Gender was included as an independent variable of interest because male gender was known to be associated with increased risk of suicide. For direct examination of the relationships between age, suicidal behavior, and cognition, the ANCOVA included a test for “parallelism” of the age regressions in each of the two groups. Detailed description of our statistical procedures has been provided elsewhere.
19,22RESULTS
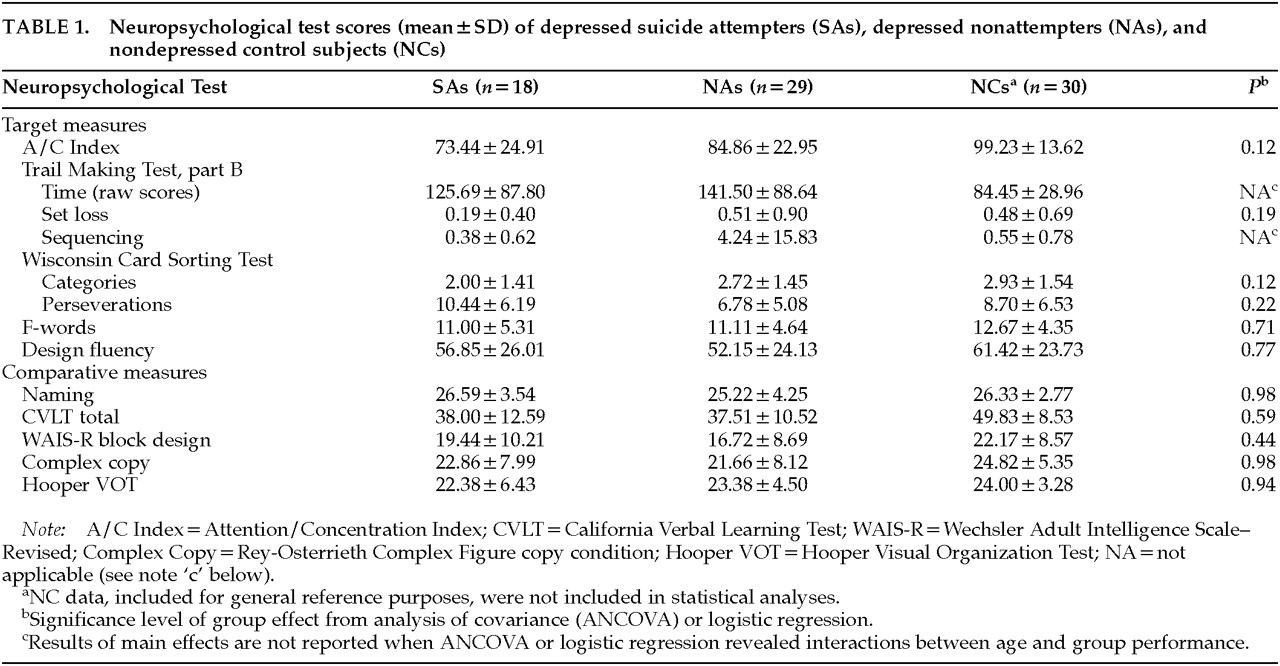
Table 1 depicts the mean scores (and standard deviations) of SAs and NAs on the neuropsychological measures, as well as the significance levels of the main effect for group from the ANCOVA. For the purpose of illustration, the table also presents the scores of 30 healthy control subjects who, as a group, matched the entire sample on age, education, gender, and Vocabulary score. These control subjects were not included in the analyses because we have reported elsewhere the neuropsychological differences between depressed patients and healthy control subjects.
19,22,35,36 Target Measures
Trails B Time data failed to meet the statistical assumptions of normally distributed errors with constant variance. Therefore, the data were log transformed for the purpose of statistical analyses. ANCOVA on the transformed data revealed an interaction between age and participant group such that the time required to complete the task increased more quickly with age in the SA group (F=6.45, df=1,34, P=0.016; R2=61.5%).
Trails B Sequencing errors did not constitute a continuous variable, but instead a binary categorization (errors versus no errors). Therefore, logistic regression was conducted, using the same ANCOVA model. The regression revealed interactions between age and group (χ=16.89, df=1, P<0.0001) and gender and group (χ=7.79, df=1, P=0.005). SAs were more likely to make errors as age increased, whereas NAs were no more likely to make errors with increasing age. Examining the interaction between group and gender, it appeared that SA males were less likely to make errors than NA males. However, very few males actually made errors (1 in the attempter group and 2 in the nonattempter group). Thus, the data regarding gender were viewed as uninterpretable.
As can be seen in
Table 1, after age, gender, and Vocabulary score were controlled, there were no differences between SAs and NAs on A/C Index (
F=2.58, df=1,41,
P=0.12); Trails B Set Loss (
F=1.81, df=1,37,
P=0.19; two outliers removed); WCST Categories (
F=2.56, df=1,22,
P=0.12); WCST Perseverations (
F=1.57, df=1,22,
P=0.22); F-Words (
F=0.14, df=1,41,
P=0.71); or Design Fluency (
F=0.08, df=1,33,
P=0.77). ANCOVA did reveal main effects of age on F-words (
F=5.42, df=1,41,
P=0.03) and Design Fluency (
F=18.60, df=1,33,
P<0.0001), such that performance declined in a parallel fashion in both groups with increasing age.
Comparative Measures
As expected, there were no significant interactions between age and group, nor any significant main effect differences between the groups on any of the comparative measures (see
Table 1). As well, there were no effects of gender. There were significant main effects of age on all of the comparative measures: naming (
F=4.78, df=1,39,
P=0.04); verbal learning (
F=36.84, df=1,42,
P<0.0001); constructional praxis (
F=22.18, df=1,42,
P<0.0001); complex copying (
F=12.82, df=1,35,
P<0.001); and visual integration (
F=49.11, df=1,41,
P<0.0001). One SA outlier who obtained an extremely low score was removed from the visual integration data without changing the nature of the results.
Supplementary Post Hoc Analyses
In light of the SAs' increasing Trail Making B Sequencing errors with age, we examined the neuropsychological testing file of each suicidal depressed patient who was at least 65 years of age to determine whether performance was impaired on Trails A (a simpler sequencing task administered as part of the larger protocol) and to determine whether there were qualitative examiner observations regarding Trails B. Of the 18 attempters, 9 ranged in age from 66 years to 84 years. Only 1 of 9 made sequence errors on Trails A. Seven of the nine test protocols contained qualitative examiner observations pertaining to performance on Trails B or other tests of complex executive function. With the exception of one individual who was noted to have “an impulsive, urgent quality to his performance,” most of the comments observed that a particular patient was slow and effortful in responding but nevertheless was able to alternate between cognitive sets (e.g., “slow, but able to demonstrate cognitive flexibility”; “slow, but without significant errors”; “generally intact attention and concentration…effortful performance on complex tasks”). Thus, it appeared that these elderly depressed attempters were effective in maintaining the cognitive set of this task at the expense of sequencing and speed.
We also sought to determine whether the Trails B findings could be caused by differences between the groups in overall medical illness. Therefore the regressions were repeated using the Cumulative Illness Rating Scale (CIRS; a valid and reliable assessment of overall medical burden based on evidence of organ system pathology
37) as a covariate. Controlling for CIRS score, time to complete the task still increased more quickly with age in the SA group (
F=5.48, df=1,33,
P=0.026). Also, SAs were still more likely to make sequencing errors as age increased, whereas NAs' sequencing performance did not change with age (χ
2=16.99, df=1,
P<0.0001).
DISCUSSION
To our knowledge, this was the first neuropsychological study of older suicidal adults. We found that depressed suicide attempters performed more poorly with increasing age than depressed nonattempters on a test of mental sequencing and flexibility (Trail Making Test, part B, Time and Sequencing). Contrary to expectation, we did not find differences between the groups on tests of attention/concentration, verbal or figural fluency, or another test of mental flexibility and abstract reasoning (Wisconsin Card Sorting Test). These findings must be considered in the context of several methodologic issues.
First, it should be clearly understood that we assessed participants after their suicide attempts (in the case of the SA group), shortly after hospitalization. It is unclear whether the very act of attempting suicide or being admitted to the hospital changed participants' affective and/or cognitive states. However, in terms of contaminating effects of the attempt itself, there was no relationship between standardized ratings of the lethality of the attempt (which included consideration of loss of consciousness) and neuropsychological performance. The effects of hospitalization could not account for the findings because both suicidal and nonsuicidal groups had been hospitalized. Moreover, the timing of cognitive assessments in this and other studies was comparable, so this could not explain differences between our findings and those of other studies reporting more pervasive differences between attempters and nonattempters.
Second, most participants in this study were using psychotropic medications. Although our previous investigation of this sample did not reveal neuropsychological effects from psychotropic use
22 and there was no difference between SAs and NAs in terms of the proportion using such medication, we lacked significant information to compare the two groups' equivalent cumulative doses of medication. Similarly, there may have been differences between SAs and NAs regarding use of nonpsychotropic medication, although our medical screening procedure excluded individuals who had clinically manifest central nervous system effects from medications.
22 Future studies should allow more detailed comparison of medication use between depressed attempters and nonattempters.
A final cautionary note is appropriate when considering the interpretation of the interactions between age, attempter status, and Trails B performance. Cross-sectional studies of age effects do not control for cohort effects; thus, we may have either over- or underestimated the effect of age.
38 Longitudinal studies of aging, depressed individuals (including suicide attempters and nonattempters) will be needed to address this problem.
Unlike some previous studies, this study did not detect differences between depressed attempters and nonattempters on tests of attention and concentration, conceptual flexibility, and abstract reasoning (WCST), or the ability to rapidly generate alternative verbal or nonverbal responses (verbal and design fluency). These negative findings are especially noteworthy given that we used larger samples than some previous studies; controlled for the effects of depression, verbal intelligence, and lethality of attempt; and used recognized and standardized diagnostic procedures to eliminate the potential contaminating effects of substance abuse, schizophrenia, or neurologic illness. Thus, our findings clearly challenge the popular cognitive-rigidity hypothesis that depressed attempters have unique impairments in a broad array of complex functions related to higher order thinking and problem-solving.
What can one conclude from the finding that performance on Trails B declined with age in the attempter group faster than in the nonattempter group in terms of both total time to complete the task and sequencing errors? Given that suicidal depressed individuals did not make more set-loss errors with age on this task, and given the nonsignificant findings on related tasks mentioned above, could these be chance findings? We conducted eight ANCOVAs, of which two (25%) were significant in a meaningfully consistent way (i.e., on the same task). If one applied the conservative Bonferroni technique to reduce the likelihood of type I error, the Trails B time finding would no longer reach significance (P=0.128), but the finding regarding sequencing errors would still be highly significant (P<0.0008). Thus, the age-related impairment on this task is unlikely to be due entirely to chance. Post hoc analysis of the performance of attempters over the age of 65 years revealed that sequencing ability was generally intact on Trails A. However, qualitative analysis of their Trails B performance suggested that they maintained cognitive flexibility on this task at the expense of sequencing and speed. That is, they were so slow and deliberate in their effort to successfully alternate between numbers and letters that they tended to “lose their place” in terms of sequence.
Rather than supporting the broader cognitive-rigidity hypothesis, these findings suggest that with increasing age there may be an association between suicidality and decreased resources for simultaneous and time-efficient processing of competing cognitive tasks (i.e., set switching and sequence tracking). Our previous work demonstrated similar age-related impairment in elderly depressed patients compared with age-matched control subjects.
19,35 The present study suggests that the problem is even more accentuated in the depressed patients who have attempted suicide. Dual-task paradigms would be helpful to directly investigate the role of age-related changes in the attentional resources of older, depressed suicide attempters.
The mechanism of the association between age, Trails B impairment, and suicidal behavior remains unclear, but the findings are consistent with the possibility that age-related changes in frontal or subcortical regions of the brain contribute to suicidal behavior. Such structures are related functionally to the modulation of complex cognitive processes.
39 Clearly, more research is needed to clarify the nature of the relationships between aging, cognition, and suicidal behavior.
ACKNOWLEDGMENTS
This research was supported by National Institute of Mental Health Grants MH40381 to Dr. Caine and MH51201 to Dr. Conwell. This work was previously presented to the American Psychological Association, San Francisco, CA, August 1998